Abstract
Background: The definition of high risk smoldering multiple myeloma (HR-SMM) is in flux. There are several models using serologic, bone marrow and radiologic data that predict for time to progression (TTP) to clinical myeloma (CMM). Lenalidomide and Dexamethasone in HR-SMM is reported to delay onset of end organ damage and improve overall survival, stressing the clinical utility of early intervention. We previously reported a GEP70 based score cutoff (<-0.26), when applied to the S0120 population, that improved the predictive power (R2) of standard clinical variables by 11%. The combination of GEP70 score >-0.26, serum M spike ≥3g/dL, and involved SFLC >25 mg/dL identified a subset of patients with 67% risk of progression at 2 years. With longer follow up, we now examine whether unique gene probe sets can be identified at the AMM stage that portend an earlier time to therapy (TTT).
Patients and Methods: We identified 105 patients with AMM who had baseline GEP data on our S0120 protocol, after IRB approval for retrospective data review, and evaluated each of 54,675 Affymetrix gene probes for their potential to predict TTT. Probes were ranked by their q-values; we found 40 probes with q-value < 0.05 and 7 probes with q-value < 0.01; the top probe had a q-value of 0.00066. Scores based on the number of significant probes at these cut-points were computed by subtracting the sum of the expressions of the up-regulated probes from the sum of the expressions of the down-regulated probes, then dividing by the total number of probes.
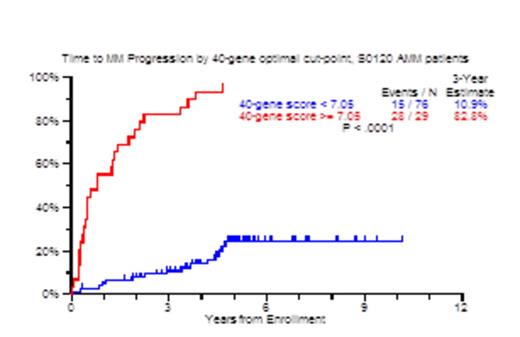
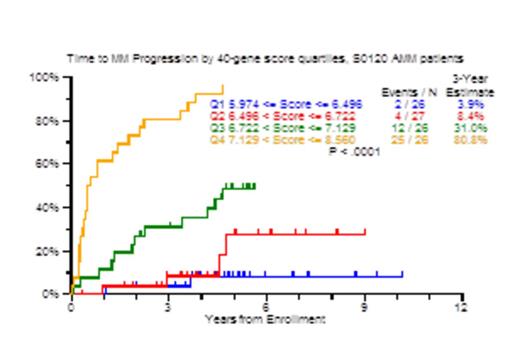
Results: In the GEP40 model, an optimal cut-point for risk of progression was identified at 7.05. The 3-year TTT probability was 83% with scores >=7.05 and only 11% for patients with values under this threshold (Figure 1A; p<0.0001). TTT probabilities also differed markedly when examined by score quartiles, attesting to a gene dose effect (Figure 1B). For the Q1 subset of 26 patients, only 4% required therapy in 3 years. Univariate Cox analysis for TTT yielded age>65 (HR: 2.3), Albumin<3.5g/dl (HR: 3.7), M-protein>3g/dl (HR: 4.99), BM plasmacytosis>=10% (HR: 12.2), GEP70>-0.26 (HR: 3.4), GEP40>=7.05 (HR: 16.41), GEP proliferation index > -0.26 (HR: 2.8), GEP PR subgroup (HR: 9.4) and GEP PolyPC >11.6 (HR: 0.22) to be significant. In the multivariate model, GEP40>=7.05 was the most significant (HR: 13.7), followed by SFLC>10mg/dl and M-protein>3g/dl. GEP40 score positively correlated with proliferation index (R: 0.804), and showed no correlation with GEP polyPC score (R: -0.156). Next, we used recursive partitioning on data from 72 patients and identified 23 patients with GEP40 score >=7.05 of whom 22 suffered TTT by 3 years (87%). Among the remaining 49 patients with GEP40 <7.0325, a further cut-point of age >59 years identified 24 patients, of whom 11 suffered progression with a 3 year TTT estimate of 25%. In the 25 patients with GEP40 <7.0325 and age <59 years, no patient progressed to CMM, with a 3 year TTT estimate of 0%. The GEP7 and GEP1 models, at optimal cut-offs, yielded equivalent positive and negative predictive values compared to the GEP40 model, albeit with less power.
Conclusion:Gene expression profiling can readily identify a subset of AMM patients who are high risk for progression to CMM. Further refinement of GEP based risk scoring can be achieved by combining clinical and correlative variables to select the super HR-SMM for intervention trials.
Zangari:Norvartis: Membership on an entity's Board of Directors or advisory committees; Onyx: Research Funding; Millennium: Research Funding. Van Rhee:Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Millennium: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Dhodapkar:Celgene: Research Funding. Morgan:Celgene Corp: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Myeloma UK: Membership on an entity's Board of Directors or advisory committees; International Myeloma Foundation: Membership on an entity's Board of Directors or advisory committees; The Binding Site: Membership on an entity's Board of Directors or advisory committees; MMRF: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.