Abstract
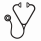
Background: MM treatment (Tx) advances have greatly improved clinical outcomes for patients (pts). A recent study demonstrated improved survival in MM through the past decade attributable to the impact of initial therapy with lenalidomide, bortezomib, and thalidomide. The greatest impact was observed in older pts (Kumar, et al. Leukemia, 2014). Connect MM, the first and largest prospective, observational, US-based, multicenter registry was designed to characterize pts, Tx patterns, and outcomes in newly diagnosed MM (NDMM).
Methods: This ongoing registry was initiated in September 2009. Eligible pts with NDMM (diagnosis must have occurred within 2 mos of study entry) were enrolled at 234 US sites. Data were collected at baseline and each subsequent quarter using an electronic case report form. The initial enrollment includes all pts who had provided informed consent as of November 1, 2012 (N = 1493). The data cutoff for this analysis was Dec 10, 2013. A total of 1444 pts were treated and were included in overall survival (OS) analyses. Survival was examined for all treated pts adjusting for pt and Tx characteristics including age, autologous stem cell transplant (ASCT) status, gender, race, disease risk factors (International Myeloma Working Group [IMWG] high risk vs. non-high risk), and therapy received (triplet vs. non-triplet) among others. Triplet therapy was defined as any combination of 3 or more drugs during the first Tx regimen. OS was estimated using Kaplan-Meier methods and comparisons across groups were assessed used the log-rank test.
Results: At the time of data cutoff, 1493 pts were enrolled with 1444 having received Tx. Of the treated pts 253 pts (18%) had IMWG high-risk disease and 108 pts (7%) had del(17p) at baseline. Median age was 67 y (range, 24-94 y), 57.2% were male, and 81.9% were white. Median follow-up was 29 mos (0-49.4 mos). The median OS for all treated pts was 44.4 mos. When assessed by age group, OS was significantly different (log-rank P < .0001) with a median of 47.6 mos for pts aged < 65 y (n = 632), 45.0 mos for those aged 65 to < 75 y (n = 443), and 33.7 mos for those aged ≥ 75 y (n = 369). OS was significantly longer for pts with ASCT vs. no ASCT (P < .0001), but not different by gender (P = .962) or race (Caucasian vs. African American vs. other; P = .250). Three-year OS probabilities by subgroup are listed in Table 1. When considering risk factors, IMWG risk was borderline significant (high vs. non-high; P = .106), and presence of del(17p) by cytogenetics and FISH was associated with significantly shortened OS (P = .005; Figure 1A). Interestingly, use of triplet therapy vs. non-triplet therapy was associated with significantly prolonged OS regardless of IMWG risk (non-high: P < .0001; high: P = .003; Figure 1B). However, no improvement was noted for triplet vs. non-triplet therapy in pts with del(17p). By multivariate analysis, the significant (P < .05) factors impacting OS were age (in 10-yr increments), International Staging System (ISS) disease stage, ECOG performance status, history of diabetes, anemia, renal function, and platelet count.
Conclusions: This interim analysis based on initially treated pts demonstrated that age, ISS stage, and co-morbidities impact OS irrespective of IMWG cytogenetic risk. Triplet Tx was associated with significantly longer OS in pts regardless of IMWG risk status. This is the largest prospective pt cohort with high-risk disease including del(17p). Pts with high-risk disease did not have significantly lower OS vs. pts without high-risk features. Pts with del(17p) (p53 deletion) continue to have shorter OS approaching 3 y and increased survival with use of triplet therapy.
Kaplan-Meier Estimated 3-Y OS Probability
| Patients . | 3-y OS Probability (%) (95% CI) . |
|---|---|
| All (N = 1444) | 62.6 (59.5-65.8) |
| < 65 y (n = 632) | 69.8 (65.2-74.3) |
| 65 to < 75 y (n = 443) | 65.0 (59.4-70.6) |
| ≥ 75 y (n = 369) | 47.2 (40.7-53.8) |
| Gender | |
| Male (n = 831) | 62.1 (57.9-66.3) |
| Female (n = 613) | 63.4 (58.7-68.2) |
| Race | |
| Caucasian (n = 1191) | 61.8 (58.3-65.3) |
| African American (n = 183) | 64.4 (55.4-73.5) |
| Other (n = 27) | 77.6 (57.3-98.0) |
| ASCT | |
| Yes (n = 494) | 77.1 (72.5-81.7) |
| No (n = 950) | 54.2 (50.0-58.3) |
| Triplet therapy | |
| Yes (n = 778) | 69.3 (65.3-73.3) |
| No (n = 666) | 54.8 (49.9-59.6) |
| IMWG risk | |
| High (n = 253) | 59.0 (51.6-66.4) |
| Standard (n = 566) | 66.3 (61.4-71.2) |
| Low (n = 86) | 75.7 (63.6-87.8) |
| del(17p) | |
| Present (n = 108) | 52.7 (41.8-63.6) |
| Absent (n = 1336) | 63.4 (60.1-66.7) |
| Patients . | 3-y OS Probability (%) (95% CI) . |
|---|---|
| All (N = 1444) | 62.6 (59.5-65.8) |
| < 65 y (n = 632) | 69.8 (65.2-74.3) |
| 65 to < 75 y (n = 443) | 65.0 (59.4-70.6) |
| ≥ 75 y (n = 369) | 47.2 (40.7-53.8) |
| Gender | |
| Male (n = 831) | 62.1 (57.9-66.3) |
| Female (n = 613) | 63.4 (58.7-68.2) |
| Race | |
| Caucasian (n = 1191) | 61.8 (58.3-65.3) |
| African American (n = 183) | 64.4 (55.4-73.5) |
| Other (n = 27) | 77.6 (57.3-98.0) |
| ASCT | |
| Yes (n = 494) | 77.1 (72.5-81.7) |
| No (n = 950) | 54.2 (50.0-58.3) |
| Triplet therapy | |
| Yes (n = 778) | 69.3 (65.3-73.3) |
| No (n = 666) | 54.8 (49.9-59.6) |
| IMWG risk | |
| High (n = 253) | 59.0 (51.6-66.4) |
| Standard (n = 566) | 66.3 (61.4-71.2) |
| Low (n = 86) | 75.7 (63.6-87.8) |
| del(17p) | |
| Present (n = 108) | 52.7 (41.8-63.6) |
| Absent (n = 1336) | 63.4 (60.1-66.7) |
Shah:Celgene Corp: Consultancy, Research Funding. Abonour:Celgene Corp: Honoraria, Speakers Bureau. Durie:Celgene Corp: Export Board Committee Other, Membership on an entity's Board of Directors or advisory committees; IRC Onyx: Membership on an entity's Board of Directors or advisory committees; DMC Millennium: Membership on an entity's Board of Directors or advisory committees; IRC J&J: Membership on an entity's Board of Directors or advisory committees. Mehta:Celgene Corp: Consultancy, Speakers Bureau. Narang:Celgene Corp: Membership on an entity's Board of Directors or advisory committees. Terebelo:Celgene Corp: Membership on an entity's Board of Directors or advisory committees. Gasparetto:Celgene: Consultancy, Honoraria; Millenium: Honoraria. Thomas:Celgene Corp: Membership on an entity's Board of Directors or advisory committees. Toomey:Celgene Corp: Membership on an entity's Board of Directors or advisory committees. Hardin:Celgene Corp: Research Funding. Srinivasan:Celgene Corp: Employment, Equity Ownership. Ricafort:Celgene Corp: Employment. Nagarwala:Celgene Corp: Employment. Rifkin:Celgene Corp: Consultancy; Millenium: Consultancy; Onyx: Consultancy; Takeda: Consultancy; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract