Abstract
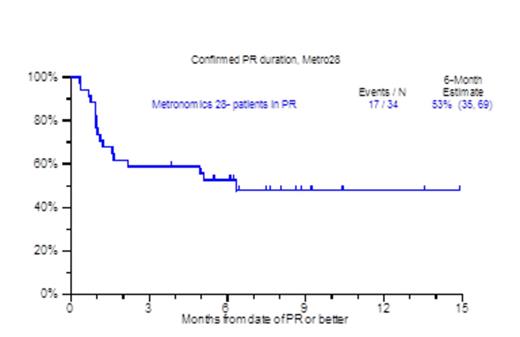
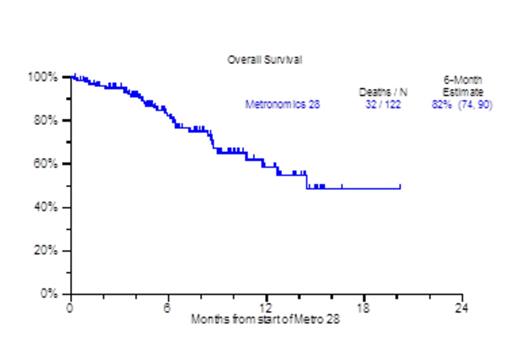
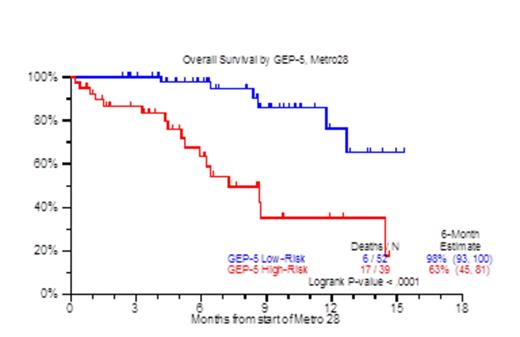
Despite the increasing availability of novel agents for RRMM, many patients run out of treatment options due to disease refractoriness and/or progressive toxicities, both hematological (cytopenia) and non-hematological (cachexia, asthenia, renal and cardiopulmonary).Patients who are refractory to both proteasome inhibitors and immunomodulatory agents (IMIDs) carry an ominous prognosis with a median overall survival (OS) of 9 months (Leukemia, Kumar 2013). We previously reported on the efficacy and toxicity profiles of a metronomically scheduled chemotherapy regimen administered over the course of 16 days (Haematologica, Papanikolaou 2014). Encouraged by the results of that treatment, we have since modified metronomic chemotherapy to cover a 28-day period (metro28). The regimen consisted largely of bortezomib 1.0mg/m2 on days 1, 4, 7, 10, 13,16,19, 22,25,28; along with dexamethasone 12mg on days 1-4, 7-10, 13-16, 19-22, 25-28; thalidomide 100mg days 1-28, continuous 28 day infusion of doxorubicin 1.0mg/m2 (0.5mg/m2 for Ejection Fraction <40%) and cis-platinum 1.0mg/m2 (0.5mg/m2 for creatinine >2mg/dL), with the addition of arsenic trioxide (ATO) at 0.1mg/kg on each day after bortezomib; and lately vincristine (VCR) at 0.07mg daily dose by continuous infusion for 28 days. Patient characteristics included age >65yr, 46%; female, 33%; cytogenetic abnormalities at any time prior to therapy (CA), 86%; Gene Expression Profile (GEP) 70-defined high risk, 44%. The median number of prior therapies was 8 (1-64), prior transplants, 83% (1, 2, >=3), 20%, 32%, 31%; prior exposure to novel agents including Carfilzomib/Pomalidomide was 81%. Thrombocytopenia prior to treatment was present in 75% including thrombocytopenia <50,000/uL in 15%. Clinical outcomes are depicted in Figure 1. At a median follow up of 8.1 months, the median number of metro 28 cycles administered was 1 (1-5), with 37% achieving a partial response and median OS not yet reached. Interestingly, the 6 month - partial response duration estimate for the patients achieving PR was 53% (Figure 1A), while the one year OS estimate for all patients receiving metro 28 was 59% (Figure 1B). Prognostic factors that held statistical significance in univariate analysis for overall survival were albumin <3.5 g/dl (HR 2.56, P=0.009), beta-2-microglobulin (B2M) ≥3.5 mg/L (HR 3.03, P=0.003), LDH≥ 190 U/L (HR 2.81, P=0.003), GEP-70 High Risk (HR 2.32, P=0.048), GEP-5 High Risk (HR 5.75, P<0.001) (Figure 1C) and GEP PR subgroup designation (HR 3.71, P=0.003). In multivariate analysis, albumin < 3.5 g/dL (HR 2.81, P=0.035), B2M≥ 3.5 mg/L (HR 3.4, P=0.017), GEP PR subgroup (HR 3.8, P=0.012), and GEP-5 High Risk (HR 8.58, P<0.001) (Figure 1C) achieved statistical significance. The majority of cycles administered in the patient population were done in the outpatient setting (81%), while secondary admissions due to regimen toxicity occurred in 36% of the cycles administered. Grade 3 & 4 neutropenia and thrombocytopenia within 3 months from the start of the metronomic treatment were evident in 64% and 78% respectively of the cases. Treatment related mortality was 2%, due to one instance each of grade 5 infection and cerebrovascular event. In conclusion 28 day metronomic therapy is a feasible treatment with acceptable toxicity and encouraging preliminary results both in terms of ORR and OS for RRMM.
Patient characteristics at start of Metro28 therapy
| Factor . | n/N (%) . |
|---|---|
| Age >= 65 yr | 56/122 (46%) |
| IgA Isotype | 28/110 (25%) |
| Female | 40/122 (33%) |
| White | 108/122 (89%) |
| Albumin < 3.5 g/dL | 47/122 (39%) |
| B2M >= 3.5 mg/L | 46/93 (49%) |
| B2M > 5.5 mg/L | 26/93 (28%) |
| CRP >= 8 mg/L | 54/122 (44%) |
| Creatinine >= 2 mg/dL | 15/122 (12%) |
| Hb < 10 g/dL | 57/122 (47%) |
| LDH >= 190 U/L | 36/121 (30%) |
| Platelet Count < 150 x 10^9/L | 92/122 (75%) |
| Cytogenetic abnormalities | 105/122 (86%) |
| CA within 1 year of therapy | 84/121 (69%) |
| CA within 90 days of therapy | 65/119 (55%) |
| New Kit GEP Sample | 91/91 (100%) |
| GEP 70 High Risk | 40/91 (44%) |
| GEP 5 High Risk | 39/91 (43%) |
| GEP CD-1 subgroup | 5/91 (5%) |
| GEP CD-2 subgroup | 17/91 (19%) |
| GEP HY subgroup | 14/91 (15%) |
| GEP LB subgroup | 6/91 (7%) |
| GEP MF subgroup | 9/91 (10%) |
| GEP MS subgroup | 9/91 (10%) |
| GEP PR subgroup | 31/91 (34%) |
| GEP proliferation index >= 10 | 28/91 (31%) |
| GEP centrosome index >= 3 | 26/91 (29%) |
| n/N (%): n- Number with factor, N- Number with valid data for factor ND: No valid observations for factor | |
| Factor . | n/N (%) . |
|---|---|
| Age >= 65 yr | 56/122 (46%) |
| IgA Isotype | 28/110 (25%) |
| Female | 40/122 (33%) |
| White | 108/122 (89%) |
| Albumin < 3.5 g/dL | 47/122 (39%) |
| B2M >= 3.5 mg/L | 46/93 (49%) |
| B2M > 5.5 mg/L | 26/93 (28%) |
| CRP >= 8 mg/L | 54/122 (44%) |
| Creatinine >= 2 mg/dL | 15/122 (12%) |
| Hb < 10 g/dL | 57/122 (47%) |
| LDH >= 190 U/L | 36/121 (30%) |
| Platelet Count < 150 x 10^9/L | 92/122 (75%) |
| Cytogenetic abnormalities | 105/122 (86%) |
| CA within 1 year of therapy | 84/121 (69%) |
| CA within 90 days of therapy | 65/119 (55%) |
| New Kit GEP Sample | 91/91 (100%) |
| GEP 70 High Risk | 40/91 (44%) |
| GEP 5 High Risk | 39/91 (43%) |
| GEP CD-1 subgroup | 5/91 (5%) |
| GEP CD-2 subgroup | 17/91 (19%) |
| GEP HY subgroup | 14/91 (15%) |
| GEP LB subgroup | 6/91 (7%) |
| GEP MF subgroup | 9/91 (10%) |
| GEP MS subgroup | 9/91 (10%) |
| GEP PR subgroup | 31/91 (34%) |
| GEP proliferation index >= 10 | 28/91 (31%) |
| GEP centrosome index >= 3 | 26/91 (29%) |
| n/N (%): n- Number with factor, N- Number with valid data for factor ND: No valid observations for factor | |
OS by GEP5
Zangari:Norvartis: Membership on an entity's Board of Directors or advisory committees; Onyx: Research Funding; Millennium: Research Funding. van Rhee:Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Millenium: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Morgan:Celgene Corp: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Myeloma UK: Membership on an entity's Board of Directors or advisory committees; International Myeloma Foundation: Membership on an entity's Board of Directors or advisory committees; The Binding Site: Membership on an entity's Board of Directors or advisory committees; MMRF: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.