Abstract
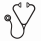
Background. AL amyloidosis is a rare acquired disease in which a monoclonal free light chain deposits as aggregated extracellular fibrils and causes organ dysfunction. Despite great progress since 2000, optimal treatment is still widely debated. Recommendations among the French network for AL amyloidosis (AL) is to first adapt treatment to disease severity evaluated by the Mayo Clinic staging (MCs) system based on cardiac biomarkers, and then on hematological responses. Stage 1 and 2 patients receive melphalan and dexamethasone (MDex). Bortezomib is added for refractory patients, after one cycle in patients with cardiac involvement (MCs 2) and after 3 cycles for patients without (MCs 1). Patients with severe cardiac disease (MCs 3) are given a combination of weekly bortezomib, oral cyclophosphamide and dexamethasone (VCD). We conducted a retrospective multicentre analysis to assess patient's outcome treated with this risk-adapted conventional strategy.
Patients and Methods. We collected data from all patients with systemic AL who had serum free light chains (FLC) and cardiac biomarkers measurements, treated in 27 French centers since January 2007 following these recommendations. Clinical parameters such as age, sex, natural history and organ involvement, as well as biological parameters, i.e FLC levels, NT-proBNP, high sensitivity troponin, creatinine etc were systematically recorded. Within the limitations of a retrospective study, treatment tolerability and toxicity data were also collected. Patients with IgM associated AL amyloidosis were excluded.
Results. One hundred and forty six patients were included and analyzed. Median age was 67 years (37-86), 42% were ³ 70, organ involvement was cardiac in 63%, renal in 70%, nerve in 24%, liver in 20%. Median dFLC at diagnosis was 200 mg/L (range 0.8 – 5301) in all patients, and increased with the severity of the disease when stratifying on Mayo stages. Median NT-proBNP increased with the Mayo staging and was 141 ng/L (0 -275) in stage 1, 867 ng/L (159-12607) in stage 2 and 8488 (1036 - 125283) in stage 3. Median creatinine serum level was 102 µmol/L (34 - 665). Median proteinuria was 2.8 g/24H (0- 24.6). Front-line treatment was M-Dex in 38 MCs 1 patients (26%) and 53 MCs 2 patients (36%) and VCD in 56 MCs 3 patients (38%). 16 patients (42%) in stage I received bortezomib and 24 (45%) of those in stage 2. Median time to switch was respectively 3.8 months (range 1.7 – 7.4) and 3.2 months (1 – 6.5) in stage 1 and in stage 2 respectively. Median number of cycles of MDex alone was 9 (range 1-13). Median number of MDex cycles before the addition of bortezomib was 3 (1-7) and median number of BMDex cycles was 4 (1 - 8) with a total median number of chemotherapy cycles in patient receiving this association of 7 (1 – 8). Stage 3 patients treated with VCD regimen receiving a median number of 2.5 cycles (range 1-11). This median number increased to 6 cycles when considering patients surviving longer than 3 months (n=37). Overall response rate for patients surviving more than 6 months and with measurable FLC with M-Dex plus or minus bortezomib (70 patients) was 89 %, including ≥VGPR in 61%, with VCD (20 patients) it was 94% including ≥VGPR in 66%. With a median follow-up for living patients of 2 years, estimated survival at 2 years for the whole population was 71% and the median OS by Kaplan-Meier analysis was not reached, the estimated 2-years survival was 80% in stage 1 and 2 versus 53% in stage 3 (p<0.0001) (Figure 1) and was 79% in patients below 70 years of age versus 60% in those older (p = 0.01). In Mayo stage 3, NT-proBNP remained a significant independent predictor of mortality. Median survival in the group with pre treatment serum NT-proBNP > 9500 ng/L or BNP > 1100 ng/L was 3 months whereas it was not reached in the group with values below these threshold with 81% estimated 2-years survival. Estimated 2-years survival in the 32 patients of our cohort treated with conventional chemotherapy according to our guidelines but who were eligible for high dose treatment according to recently published criteria (Gertz MA, Am J Hematol. 2013;88:416-25: age<70, NT-proBNP<5000 ng/l, cTnT< 0.06 µg/L, creatinine ≤150 µmol/L and organ involvement<3) was 87%.
Conclusion. In AL, a simple risk-adapted and response-tailored treatment, excluding ASCT, can give a high response rate and a very good survival even in a multicenter setting.
Jaccard:Janssen Cilag: Honoraria; Celgene: Honoraria, Research Funding. Tournilhac:mundipharma: Honoraria, Other, Research Funding; GSK: Honoraria, Other, Research Funding; Roche: Honoraria, Other, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract