Abstract
Background: Overcoming the block of differentiation is a major effort in Acute Myeloid Leukemia (AML) research. We visited the major quantitative trait locus (QTL) for hematopoietic differentiation on chromosome 6q23 to identify genes involved in hematopoietic development that are possibly deregulated during leukemogenesis. We noticed a novel microRNA within the QTL: miR-3662.
Aims: The goal was to elucidate a possible causal involvement of miR-3662 in the association of the 6q23 QTL with hematopoietic differentiation and/or leukemogenesis.
Results: Monitoring of miR-3662 expression during cytokine stimulated differentiation of CD34+ hematopoietic progenitor cells (HPCs) revealed increasing miR-3662 abundance during differentiation, with the highest expression found in terminally differentiated cells. In contrast, miR-3662 had very low expression in AML cell lines (KG1a, MV4-11, OCI-AML3, THP1) and primary AML patient blasts (n=12).
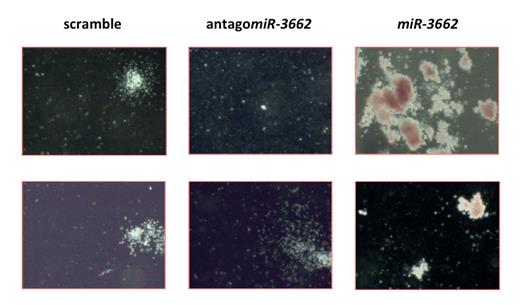
We stably introduced miR-3662 into HPCs of 3 non-leukemic donors, and differentiated the cells into erythroid, megakaryocytic and granulocytic lineages. Forced expression of miR-3662 led to significant increase of colony formation, most pronounced in the erythroid lineage. In contrast, targeted knock-down of miR-3662 significantly reduced colony formation (Figure 1). To test if the observed downregulation of miR-3662 in leukemic cells is favorable to leukemic cells, we stably introduced miR-3662 in AML cell lines (MV4-11, KG1a) and AML patient blasts (n=12). Increasing the abundance of miR-3662 led to reduction of cell viability, increased cell death and reduced colony formation.
To find the downstream targets of miR-3662, we performed a comprehensive targeted RNA sequencing approach with a panel of 361 genes (TruSeq platform, Illumina; followed by Ingenuity Pathway analysis), using RNA from two AML patient blasts infected with miR-3662 vs. scramble. miR-3662 was associated with >20% reduction in the mRNA expression of 34 genes. Among these, 6 genes harbored predicted miR-3662 binding sites in their 3′-UTRs, qualifying as potential direct miR-3662 targets. Of these, the inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase beta (IKBKB) had the highest affinity score for miR-3662, and was thus chosen as the primary candidate.
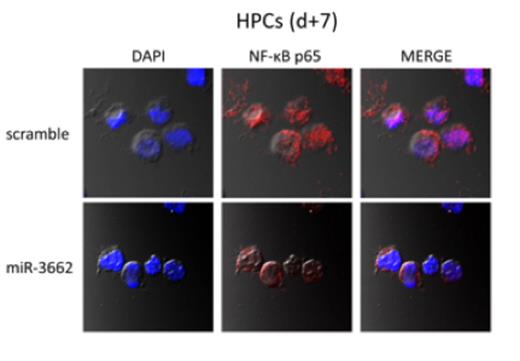
Determination of endogenous miR-3662 and IKBKB expression in primary AML patient blasts (n=12) showed a significant association of higher miR-3662 with lower IKBKB abundance. qPCR and Western blotting confirmed miR-3662 mediated downregulation of IKBKB in HPCs, KG1a and MV4-11 cells and primary patient blasts. Because IKBKB leads to reduced phosphorylation of the inhibitor in the inhibitor/NF-ĸB complex (IKBa), it limits NF-ĸB's nuclear localization and consequent transcriptional activation. Increasing miR-3662 should thus decrease NF-ĸB's nuclear localization. Western blotting showed reduced phosphorylation of IKBa and confocal imaging showed less NF-ĸB in the nucleus in miR-3662 expressing MV4-11 and HPC cells (Figure 2). Luciferase assay validated a direct interaction of miR-3662 with the 3′-UTR of IKBKB.
Finally, we attempted to gain insights into the upstream regulation of miR-3662. We identified a possible transcription start site for miR-3662 (TSS-3662), whose activation potential was validated by both luciferase assays and enrichment of RNA polymerase II (Pol II), histone H3 methylated Lys (H3K4), and total histone H3 CHIP assays of TSS-3662. Testing of predicted activating transcription factors showed GATA1 and CEBPA to have an activating potential (as shown by luciferase assays) and binding affinity (as shown by electrophoretic mobility shift assays) to TSS-3662. Overexpression of GATA1 and CEBPA significantly increased miR-3662's abundance.
Conclusion: We have identified miR-3662, located in the major hematopoietic QTL on chromosome 6q23, as a novel regulator of hematopoietic differentiation that acts by regulating the NF-ĸB pathway. Reduced abundance of miR-3662 in leukemic cells potentially contributes to the de-differentiated phenotype of leukemic cells and to increased cell growth by increasing nuclear localization of NF-ĸB.
Effect of miR-3662 overexpression and knock-down on colony formation of HPCs.
Cellular localization of NFĸB in miR-3662 vs. scramble-infected HPCs.
Walker:Karyopharm Therapeutics Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.