Abstract
Survival (OS) in elderly patients with AML is poor. Azacitidine (AZA) prolongs OS in elderly patients even with >30% marrow (BM) blasts (Dombret, EHA, 2014). Remarkably, a hematologic response to AZA, not only complete remission (CR), correlates with a survival advantage. Yet, AZA and induction chemotherapy (IC), which could induce a 50% response in patients > 60 years, are not mutually exclusive. Identifying biomarkers for response to AZA such as BM blasts > 45% on day (d)15 of the first cycle, and response at d56 might identify patients early who are less likely to have a long-term benefit from AZA and for whom IC might be an option (Al-Ali et al, Leuk Lymph, 2012). Indeed, in vitro data suggest a possible synergistic effect of cytarabine and AZA when AZA is administered before cytarabine (Neil et al, Cancer Res, 1976; Kong et al, Mol Pharmacol, 1991). Results of the phase I of the DRKS00004519 (RAS-AZIC) study are presented where the feasibility of priming with AZA prior to IC was determined.
Patients and methods: RAS-AZIC is a prospective, multicentric, phase I/II trial evaluating the efficacy of priming with AZA followed by a sequential, and response-adapted therapy on d17, d56, and d90 with AZA or IC in eligible patients > 60 years with newly diagnosed AML (phase II). AZA is continued on d28 if d15 BM blasts are < 45%, or else, IC on d17 is given. If response at d56 is CR/CRi, maintenance with AZA is continued/started. Otherwise, IC is given followed by AZA maintenance on d90 in patients who achieve CR/CRi/partial remission. Patients in CR could be assigned to allogeneic stem cell transplantation.
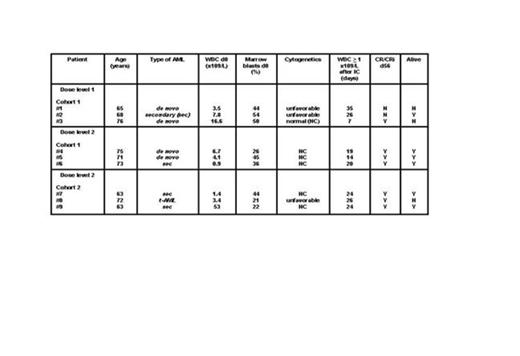
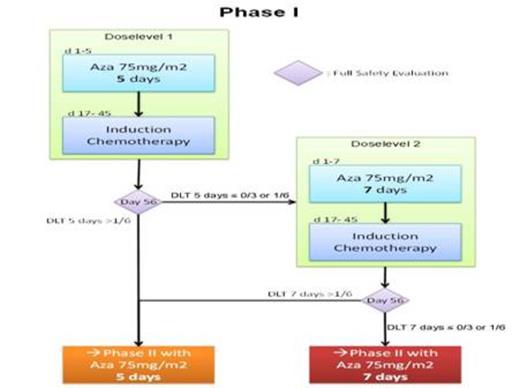
In the monocentric phase I part, the safety and dose level of priming with AZA 75 mg/m2/day s.c. for 5 (dose level 1) or 7 days (dose level 2) followed by IC on d17 (Mitoxantrone 10 mg/ m2/day d 1-3 and cytarabine 1 g/ m2/BID d1, 3, 5, 7) needed to be established through a 3+3 design. Six to 12 patients were required with at least 3 patients treated at dose level 1 (figure 1). The level at which not more than one of 6 patients experienced a dose limiting toxicity (DLT) would be used in the phase II. DLT was defined as a medical event, at least possibly related to trial therapy, occurring within 56 days, and fulfilling one of the following: Grade ≥ 3 liver or renal toxicity (NCI CTCAE 4.03), or delay in WBC regeneration (≥ 1x109/L) beyond d45 after IC. After each cohort, a safety evaluation by an independent Data Monitoring Committee (DMC) was performed. All pts gave written informed consent.
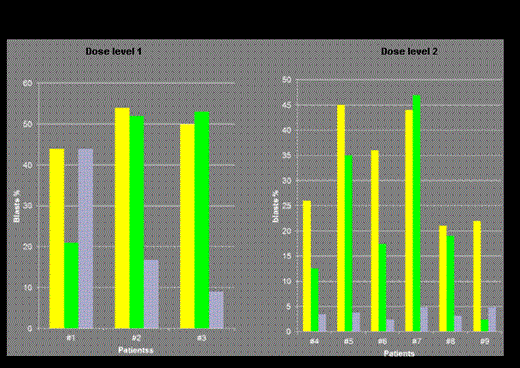
Results: The first 3 patients were enrolled at dose level 1. No DLT was encountered. Again, at dose level 2, no DLT occurred in cohort 1 and 2 (0/6 patients). Thus, phase I was completed after enrolling 9 patients. Median age was 71 (range 63-76) years, 5 patients (56%) were female. Secondary/therapy-related AML, and unfavorable cytogenetics were present in 56%, and 33% of patients respectively. Baseline median WBC and marrow blasts were 4.1 x109/L and 44% respectively (Table). AZA priming was given as out-patient therapy in 67% of patients. In 8 (89%) patients, IC was started as per protocol. In patient #9, IC was delayed and given on d26 because of viral pneumonia. Median WBC regeneration ≥ 1 x109/L following IC occurred after 24 (range 7-35) days. No early death on d90 ensued. CR/CRi on d56 in the entire phase I, and dose level 2 was 78%, and 100% respectively. BM blasts > 45% on d15 were documented in two patients at dose level 1, and one patient at dose level 2 (Figure 2). To date and after a median follow-up of 13 (range 6-19.5) months, median survival time is 12 (range 4-19) months with a survival rate of 67% . Priming with AZA 75 mg/m2/day s.c. for 7 days was considered safe and feasible by the DMC and ethic board. Phase II has now been initiated.
Conclusions: Priming with AZA followed by induction chemotherapy is feasible in elderly pts with newly diagnosed AML. Results of the phase I are very encouraging in terms of safety and efficacy. It is unlikely that a single agent alone could accomplish the goal of attaining rapid responses and translating them into long-term survival in all pts because of the heterogeneity of the disease. Integrating AZA epigenetic therapy with chemotherapy in well-designed trials might further optimize outcome in elderly patients.
Al-Ali:Celgene: Honoraria, Research Funding. Off Label Use: azacitidine priming prior to chemotherapy in AML within a clinical trial.
Author notes
Asterisk with author names denotes non-ASH members.