Abstract
Introduction: Leukemic stem cells (LSCs) are recognized as important mediators of chemotherapy resistance and leukemia relapse. The postulated mechanism for this is the relative quiescence of these cell populations that renders them resistant to cytotoxic agents. This simple hypothesis, however, is supported almost entirely by indirect evidence, and fails to explain the large differences in relapse rates across different AML subtypes. To address this question, we have developed a mass cytometry (MCM) approach to assess the cell cycle of immunophenotypically complex primary samples from patients with AML. By processing samples immediately upon bone marrow harvest, we could determine if AML stem cells were quiescent in vivo and if the cell cycle properties of these cells varied between chemotherapy-responsive versus resistant AML subtypes.
Methods: Bone marrow aspirates from 33 AML patients, 3 with APL, 2 with high-risk MDS, 5 with AML who achieved a CR with chemotherapy treatment, and 5 healthy donors (48 total samples) were incubated at 37°C for 15 minutes with 20uM Iodo-deoxyuridine (IdU) immediately after aspiration (<1 min), followed by fixation and storage. Samples were then analyzed with two overlapping 39-antibody MCM panels (50 markers total). Cellular barcoding was utilized to stain and analyze cells in tubes of 20 samples each, enabling direct comparison of samples to each other and to the healthy controls.
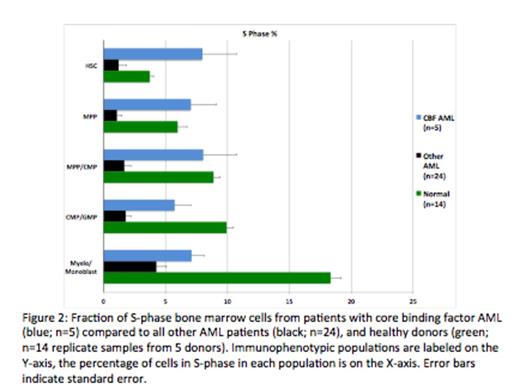
Results: The high dimensionality of MCM enabled the simultaneous measurement of 25 surface markers and the identification of almost all immunophenotypic populations in human bone marrow. The use of barcoding, and the resultant ability to directly compare samples, enabled the detection of aberrant marker expression at very high resolution (2-3 fold changes). At least one surface marker aberrancy was detected in each AML sample. Unexpectedly, cell cycle analysis revealed that, compared to immunophenotypically similar normal cells, the average fraction of S-phase cells in AML samples was significantly lower. In both AML and healthy samples, the lowest S-phase fraction was found in fully differentiated populations and in hematopoietic stem cells (HSCs) while committed progenitor populations (myelo-monoblasts, promyelocytes, erythroblasts) exhibited the highest S-phase fraction. The HSC and early progenitor cell populations from patients with CBF AML (t(8;21) and inv(16)) demonstrated a significantly higher S-phase fraction than the same cell populations from the other AML samples (7.76% vs. 2.66%; p=0.0014). Furthermore, samples with FLT3-ITD mutations exhibited the lowest S-phase fraction in the HSC and early progenitor cell populations (0.63%), which was significantly lower than the S-phase fraction of the other AML samples (4.37%; p=9.3x10-4).
Finally, a subset of patients (n=10) was being treated with hydroxyurea (HU) at the time of their bone marrow aspiration. The effect of HU treatment was manifest as a reduction in the IdU incorporation rate (with no change in S-phase fraction) in the cells of the treated patients. However, neither cell cycle arrest nor apoptosis were observed in these samples. This is in contrast with the commonly observed occurrence of both in leukemic cell lines treated in vitro with HU.
Conclusions: By combining fresh sample processing with high-dimensional MCM analysis, we developed an innovative approach for the analysis of hematologic malignancies. Our results suggest that the relative sensitivity of CBF AML to cytotoxic chemotherapy may be the result of the increased fraction of S-phase cells within the HSC and early progenitor cell populations. Conversely, HSC and early progenitor cell populations from patients with FLT3-ITD mutations would be expected to be particularly resistant to cytarabine-based consolidation therapy due to the very low frequency of S-phase cells within these populations. This finding, combined with our observation that the stem and early progenitor cells from the FLT3-ITD samples have high expression of CD33, may provide a mechanistic explanation for the improved disease-free survival recently reported for FLT3-ITD AML patients treated with fractioned gemtuzumab ozogamicin in combination with standard therapy.
Behbehani:Fluidigm: Consultancy. Medeiros:Agios: Consulting - Ad board Other. Nolan:Fluidigm, Inc: Consultancy, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.