Abstract
Background: The FAB classification of AML was based on the differentiation state and the involved cell lineages and recognized some close associations between genetic abnormalities and cytomorphology: CBFB-MYH11 and AML with abnormal eosinophils, PML-RARA and acute promyelocytic leukemia; RUNX1-RUNX1T1 and AML with maturation (FAB subtype M2). Associations with molecular mutations have not been examined in detail yet.
Aim: Evaluate the impact of molecular mutations on the AML differentiation state.
Material and Methods: The cohort comprized 3,703 patients with de novo AML (median age 67 yrs; range: 18.1-100.5 yrs). All cases were analyzed by cytomorphology and molecular mutation screening for 12 genes by next generation or Sanger sequencing in our laboratory. Based on cytomorphology and immunophenotyping we categorized the cohort into 11 subgroups: 1. AML with minimal differentiation (according to the FAB classification: M0; n=192), 2. AML without maturation (M1; n=1,015), 3. AML with maturation (M2; n=1,324), 4. acute promyelocytic leukemia (APL) with PML-RARA (M3; n=124), 5. the microgranular variant of APL (M3v, n=110), 6. acute myelomonocytic leukemia (M4; n=604), 7. acute myelomonocytic leukemia with abnormal eosinophils and CBFB-MYH11 (M4eo; n=81), 8. acute monoblastic leukemia (M5a; n=86), 9. acute monocytic leukemia (M5b; n=63), and 10. acute erythroid leukemia (M6; n=101). Cases with acute megakaryoblastic leukemia (M7; n=3) were not considered for statistical evaluation. All 3,703 cases were analyzed for FLT3-ITD and FLT3-TKD. All cases except M3/M3v and M4eo (n=3,487) were screened for NPM1 und MLL-PTD. Data on the mutation status of the following genes was available: FLT3-ITD (n=3,703), FLT3-TKD (n=3,703), CEBPA (n=2,684), RUNX1 (n=2,320), WT1 (n=2,224), ASXL1 (n=1,591), DNMT3A (n=1,294), NRAS (n=1,232), TP53 (n=1,046) and TET2 (n=974). Statistical significance of associations between the mutation frequencies and AML subtypes were assessed by Fisher’s exact test and corrected according to Benjamini-Hochberg (1995) to compensate for multiple testing.
Results: The mutation rates of the analyzed genes are in line with published data.
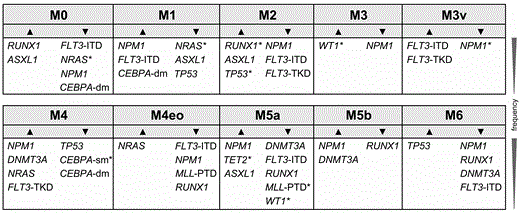
Total cohort: Significant associations were observed between the mutation frequencies of distinct genes and the differentiation stage of the AML. Figure 1 depicts genes showing significantly different mutation rates within distinct AML subtypes compared to the rate of the total cohort. In detail, mutations in RUNX1 were most frequently observed in M0 (43.6%) and M2 (24.0%) and were nearly absent in M5a (3.5%) and M5b (0%). The highest TP53 mutation rates were observed in M6 (35.3%) and M2 (15.0%), the lowest in M4 (3.0%) and M1 (5.7%). NPM1 mutations were significantly more frequent in M5b (69.8%), M4 (56.3%), M5a (47.7%) and M1 (42.1%) than in M2 (24.2%), M6 (20.8%), M0 (3.1%), and absent in M3, M3v, and M4eo, respectively.
NPM1 mutated subcohort (n=1,200): The overall DNMT3A mutation rate in this subcohort was 49.1%. It was significantly higher in M5b (75.0%) and M4 (61.6%) and significantly lower in M1 (40.6%), M5a (22.2%), and M6 (13.3%). Contrary, RUNX1 (overall frequency in NPM1mut cases 1.2%) and ASXL1 (overall 2.5%) mutations were more frequent in M5a (8.3% and 34.8%, respectively), while FLT3-ITD was more frequent in M1 (52.5%), and WT1 mutations more frequent in M6 (26.7%).
CEBPA double mutated subcohort (n=108): ASXL1 and RUNX1 mutations were 5- and 7-fold more frequent in M2 than in M1, but did not reach significance.
Conclusions:
Specific patterns of molecular mutations are linked to distinct AML differentiation stages: 1) RUNX1 and ASXL1 with M0 2) NPM1, ASXL1 and TET2 with M5a, and 3) NPM1 and DNMT3A with M5b. 4) The differentiation stage of NPM1 mutated AML is driven by the presence of additional mutations: DNMT3A mutations lead to a (myelo-)monocytic differentiation, while mutations in RUNX1 or ASXL1 lead to a monoblastic phenotype. 5) FLT3-ITD is most frequent in NPM1 mutated AML without maturation and WT1 mutations are most frequent in acute erythroid leukemia bearing NPM1mut.
Morphologic classes and the identified genes with significantly different mutation rates compared to the total cohort: ▲ genes with a higher mutation frequency; ▼ genes with lower mutation rates (significance levels were corrected according to Benjamini-Hochberg, * indicates trends (0.05<p<0.13,), for all other p<0.05)
Morphologic classes and the identified genes with significantly different mutation rates compared to the total cohort: ▲ genes with a higher mutation frequency; ▼ genes with lower mutation rates (significance levels were corrected according to Benjamini-Hochberg, * indicates trends (0.05<p<0.13,), for all other p<0.05)
Rose:MLL Munich Leukemia Laboratory: Employment. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Schnittger:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Perglerová:MLL2 s.r.o.: Employment. Kern:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.