Abstract
Background: Umbilical cord blood transplantation (UCBT) has emerged as an alternative for allogeneic hematopoietic stem cell transplantation for patients who don't have a matched donor. However, cell dose remains an obstacle to successful UCBT and strategies aimed at improving rate and speed of engraftment are needed. Acid sphingomyelinase (ASM), a glycoprotein enzyme that converts sphingomyelin to ceramide, was recently shown to modulate inflammatory and cell stress responses. In vitro treatment with docosahexaenoic acid (DHA), an ASM inhibitor, restores colony formation ability of CD34+ cells from diabetic patients and that paralleled reduction of ASM in such cells (Tikhonenko et al. PLoS One 2013). Fingolimod (FTY720) is a novel immunosuppressant that is FDA approved for the treatment of relapsing multiple sclerosis. Fingolimod was shown to inhibit ASM activity in vitro through a mechanism that is similar to desipramine (a known inhibitor of ASM) yet at much lower micromolar concentrations (Dawson et al. Biochem Biophys Res Commun 2011).
Hypothesis: Fingolimod expands UCB cells and improves engraftment of UCB cells in NSG mice.
Methods: CD34+ cells, separated by magnetic-activated cell sorting (MACS) from pooled human UCB units, were cultured for 72 hours at 37 °C in RPMI-1640 + 10% FBS + 100 ng/ml each of SCF, TPO, and FL. Cells were be divided into 3 plates; the third plate had fingolimod added to culture (at 1 uM concentration) and the first 2 plates had no fingolimod added. Sphingomyelinase activity was measured using the Amplex Red Sphingomyelinase Assay Kit according to the manufacture protocol (Molecular Probes, Eugene, OR). Cells were then plated into methylcellulose for 7-10 days before colony forming units (CFU) were counted. Cells from each plate were injected into a corresponding group of sublethally irradiated (225cGy) NSG mice (n=5 per group) as follows: group 1 was injected with control cells, group 2 was injected with control cells, but mice received 5 mcg/kg/day fingolimod by intraperitoneal injection starting on the day of transplant for 6 weeks, and group 3 was injected with cells cultured with fingolimod. Engraftment of human cells was measured by flow cytometry for CD45 surface marker in peripheral blood of recipient mice at 3 weeks and 8 weeks. Engraftment rate of human UCB cells in NSG mice was estimated using a mixed statistical model.
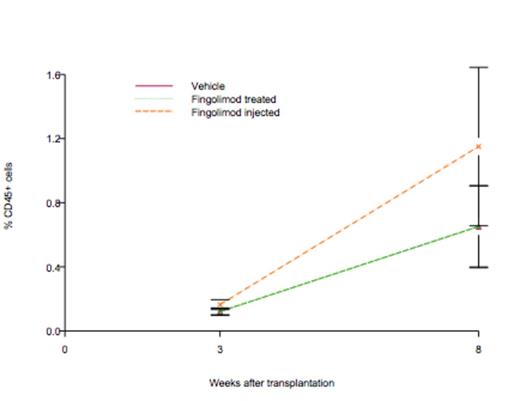
Results: Adding fingolimod to cell culture resulted in a robust expansion of UCB cells as evident by doubling in CFU (p<0.0001). Fingolimod also decreased acid, but not neutral sphingomyelinase activity by 25%. In NSG mice, the engraftment rate for control group was 0.106, which was not statistically significant from zero (p = 0.104). The slope for fingolimod treated group was 0.106, which was not significant from zero, either (p = 0.104). However, the slope for fingolimod injected group was 0.197, which was significant from zero (p = 0.0066) as shown in figure below.
Conclusions: Fingolimod expands CFU activity of human UCB cells in culture. In addition, in vivo treatment of NSG mice with fingolimod accelerates engraftment of human CD34+ selected UCB cells.
In vivo treatment of NSG mice with fingolimod accelerates engraftment of human UCB cells. Rate of engraftment estimated using a mixed statistical model (p=0.0066).
In vivo treatment of NSG mice with fingolimod accelerates engraftment of human UCB cells. Rate of engraftment estimated using a mixed statistical model (p=0.0066).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.