Abstract
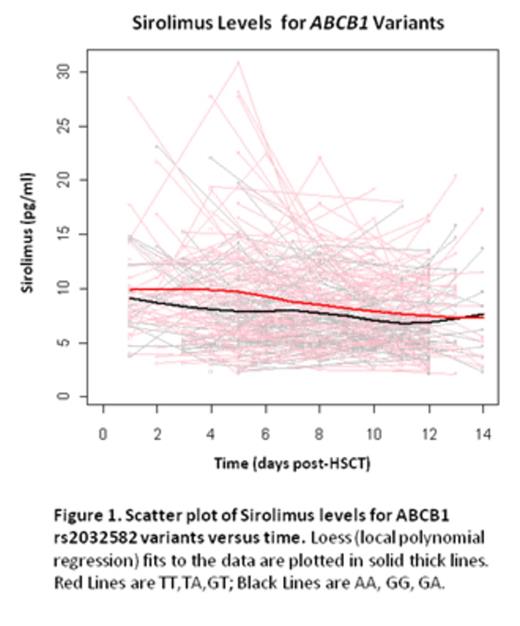
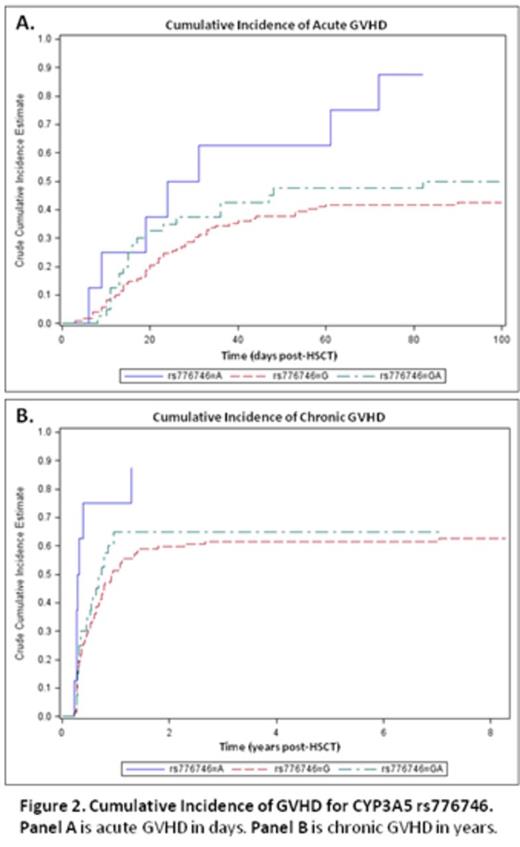
Allelic variants implicated in drug absorption, distribution, metabolism, and excretion (ADME) affect drug pharmacokinetic variability and have been increasingly recognized as important factors in medical therapy. In solid organ transplantation, certain ADME genes affect the blood level of immunosuppressants as well as clinical outcomes despite rigorous traditional therapeutic drug monitoring (TDM). The influence of ADME pharmacogenetics in Hematopoietic Stem Cell Transplantation (HSCT) has not been well studied, with few publications and none reporting the effects in the setting of the tacrolimus (TAC)/ sirolimus (SIR) GVHD-preventive combination. In this exploratory pilot study, the objective was to evaluate possible associations between ADME variants with TAC/SIR blood levels and clinical outcomes in the allogeneic HSCT setting. The primary focus was on 3 ADME genes [ABCB1 (MDR1), CYP3A4, and CYP3A5] known to influence TAC/SIR outcomes in solid organ transplant. Secondarily we explored associations with other gene variants on the ADME panel. We analyzed archived DNA samples from 179 HSCT recipients on the Sequenom MassARRAY® platform. This panel is based on the PharmADME Working Group core list and interrogates 184 allelic variants and 12 copy number variants and 4 gene conversions (in 36 pharmacogenetically relevant genes). Blood levels of TAC and SIR were collected for all patients at least once weekly for the first 100 days post-transplant. For this analysis, median blood levels of TAC and SIR were obtained for the first 7 and 14 days post-transplant. Conditioning regimens consisted of fludarabine/melphalan (n=106), total body irradiation (TBI)/cyclophosphamide (n=11), TBI/etoposide (n=46) and busulfan/cyclophosphamide (n=14). All patients received TAC/SIR-based GVHD prophylaxis according to Shayani et al. (Biol Blood Marrow Transplant 2013). No azoles were used as fungal prophylaxis. Of 179 samples genotyped, 178 showed high quality data. The average call rate for these samples was 98.85% over 200 assays, with a median call rate of 100%. Of these assays, 66 variants were identified that could be evaluated for association with TAC/SIR levels and clinical outcomes; other assays were excluded due to homozygosity or >10% missing data. In the setting of the TAC/SIR combination, the median SIR blood level over the first 14 days post-HSCT was higher in rs2032582 (ABCB1) T carriers vs other groups (p=0.01), as was the median concentration/dose (C/D) ratio (p=0.05) (Table). We also found that the median TAC blood level over the first 7 days post-HSCT was lower in the rs776746 ( CYP3A5) AA group compared to GG or GA groups (p<0.002), as well as for TAC C/D ratio (p=0.01). To evaluate effects of gene variants on drug levels over time, the generalized estimating equation (GEE) approach were used to facilitate the analysis; results showed a significant difference in SIR levels between rs2032582 (ABCB1) T-carriers vs other groups. This is consistent with our observation that initial genetic influences on drug levels lessen over time due to TDM dose adjustments (Fig 1). Despite TDM dose adjustment, the presence of the CYP3A5 AA polymorphism (fast drug metabolizers) was associated with increased incidence of acute and chronic GVHD (Fig 2). None of the SNPs for the 3 primary genes tested were significantly associated with renal dysfunction or thrombotic microangiopathy. Our exploratory analysis identified multiple other genetic variants potentially associated with TAC / SIR levels and clinical outcomes, but none reached statistical significance after adjustment for multiple testing. These variants will be included in a focused panel for future validation. Our study is the first to demonstrate the influence of ADME genetic variants on drug levels and clinical outcomes after HSCT using TAC/SIR as GVHD prophylaxis. Our results may lead to individualized dosing of immunosuppressive medications post-HSCT based on ADME genetic polymorphisms.
| SIR Drug Level First 14 Days | SIR C/D Ratio First 14 Days | |||||||||
| Gene | SNP | Group | N | Median | Range | p-value | N | Median | Range | p-value |
| ABCB1 | rs2032582 | AA/GG/GA | 60 | 6.9 | 2.1-13.1 | 0.01 | 60 | 1.9 | 0.6-12.3 | 0.05 |
| TT/TA/GT | 112 | 8.3 | 2.8-18.7 | 112 | 2.2 | 0.8-5.4 | ||||
| TAC Drug Level First 7 Days | TAC C/D Ratio First 7 Days | |||||||||
| Gene | SNP | Group | N | Median | Range | p-value | N | Median | Range | p-value |
| CYP3A5 | rs776746 | AA | 8 | 9.3 | 5.0-16.8 | <0.002 | 8 | 7.7 | 5.5-13.3 | 0.01 |
| GG | 122 | 11.0 | 3.1-27.0 | 122 | 10.6 | 4.9-33.8 | ||||
| GA | 40 | 9.4 | 3.9-16.2 | 40 | 8.2 | 2.8-16.4 | ||||
| SIR Drug Level First 14 Days | SIR C/D Ratio First 14 Days | |||||||||
| Gene | SNP | Group | N | Median | Range | p-value | N | Median | Range | p-value |
| ABCB1 | rs2032582 | AA/GG/GA | 60 | 6.9 | 2.1-13.1 | 0.01 | 60 | 1.9 | 0.6-12.3 | 0.05 |
| TT/TA/GT | 112 | 8.3 | 2.8-18.7 | 112 | 2.2 | 0.8-5.4 | ||||
| TAC Drug Level First 7 Days | TAC C/D Ratio First 7 Days | |||||||||
| Gene | SNP | Group | N | Median | Range | p-value | N | Median | Range | p-value |
| CYP3A5 | rs776746 | AA | 8 | 9.3 | 5.0-16.8 | <0.002 | 8 | 7.7 | 5.5-13.3 | 0.01 |
| GG | 122 | 11.0 | 3.1-27.0 | 122 | 10.6 | 4.9-33.8 | ||||
| GA | 40 | 9.4 | 3.9-16.2 | 40 | 8.2 | 2.8-16.4 | ||||
Khaled:Sequenom: Research Funding. Off Label Use: Use of tacrolimus and sirolimus immunosuppressants for prevention of graft-versus-host disease following allogeneic hematopoietic stem cell transplantation..
Author notes
Asterisk with author names denotes non-ASH members.