Abstract
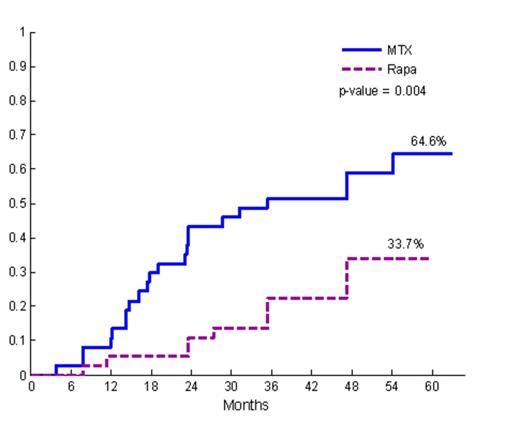
Current pharmacologic strategies employed in allogeneic hematopoietic cell transplantation (HCT) largely fail to prevent chronic graft vs. host disease and do not effectively induce donor-recipient immune tolerance. We report a long-term follow-up (LTFU) analysis of a randomized phase II trial comparing sirolimus/tacrolimus (SIR/TAC, n=37) vs. methotrexate/tacrolimus (MTX/TAC, n=37). The primary endpoint of the trial was grade II-IV acute GVHD within 100 days. Per protocol, SIR/TAC patients were treated with at least 1 year of SIR post-HCT. In the present LTFU analysis, we studied the following: Compliance with SIR; incidence, severity, classification, and organ manifestations of chronic and late acute GVHD; glucocorticoid exposure; discontinuation of immune suppression (IS); and current estimation of relapse, non-relapse mortality (NRM) and overall survival. A subset analysis examined chronic GVHD therapeutic outcomes. The study groups had no significant differences in recipient/donor age or gender, diagnosis and remission status, CIBMTR risk category, donor type, or conditioning regimen (p=NS). The cumulative incidence of grade II-IV acute GVHD through day 100 post-HCT differed (SIR/TAC: 43% vs. MTX/TAC: 89%, p < 0.001). Median follow up time for surviving patients was 41 months (range 27-60) for SIR/TAC and 49 months (range 29 – 63) for MTX/TAC. One patient discontinued SIR at 161 days post-HCT due to TMA (grade 1). Otherwise, SIR was not discontinued for toxicity, and the median duration of SIR therapy among patients (n=27) surviving ≥ 1 year was 33 months (range 5.29 – 60 months). NIH Consensus moderate-severe chronic GVHD was significantly lower in SIR/TAC vs. MTX/TAC (34% vs. 65%, p=0.004). NIH global (0-3) severity score was significantly lower in SIR/TAC vs. MTX/TAC (p=0.003). While not significantly different, lung, liver, and GI involvement and severity were decreased in the SIR/TAC group, and fewer cases had overlap subtype of chronic GVHD (7 vs. 14, p=0.15). Late acute GVHD was also significantly lower among SIR/TAC vs. MTX/TAC (20% vs. 43%, p=0.04). While SIR/TAC patients had lower prednisone exposure and earlier discontinuation of tacrolimus (median time to TAC discontinuation SIR/TAC 368 days vs. MTX/TAC 821 days, p=0.002), there was no difference in complete IS discontinuation (at 60 months post-HCT: SIR/TAC 43% vs. MTX/TAC 31%, p=0.78). Malignancy relapse was lower among SIR/TAC vs. MTX/TAC (19% vs. 39%, p=0.06), and overall survival was not different. Co-administration of escalated dose IV busulfan (7500µM/L*min/day) together with fludarabine resulted in excess NRM among SIR/TAC (NRM: 3/5) patients, but not MTX/TAC (NRM: 1/11). When restricted to standard dose conditioning, no difference in NRM was observed (at 48 months: SIR/TAC 10% vs. MTX/TAC 16%, p=0.98). Subgroup analysis of those with chronic GVHD did not demonstrate differences (second-line systemic IS therapy, treatment success, or failure-free survival) to suggest that ongoing SIR treatment modified the natural history and therapeutic responsiveness of chronic GVHD. In summary, prolonged sirolimus administration is associated with reduced moderate-severe chronic and late acute GHVD, decreased total prednisone exposure, and earlier TAC discontinuation post-HCT. Investigation of determinants of immune suppression discontinuation and development of tools to assess immune tolerance are needed to advance the field.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.