Abstract
Background
Mantle cell lymphoma (MCL) is an aggressive subtype of NHL with a poor prognosis, with few effective treatment options in the relapsed setting. Ibrutinb (Imbruvica"), a first-in-class, once daily, oral covalent inhibitor of Bruton's Tyrosine Kinase (BTK) was recently approved in the US for treatment of MCL in patients who have received at least one prior therapy, based on a Phase II study (Wang et al., 2013; PCYC-1104) in patients with relapsed or refractory (r/r) MCL. Overall, the patients receiving ibrutinib experienced a median progression-free survival (PFS) of 13.9 months and overall response rate (ORR) of 68%, and 58% of patients were alive at 18 months. Here we assessed the clinical effectiveness and project long-term outcomes of ibrutinib against other commonly used treatment options in r/r MCL through a simulation model. Methods
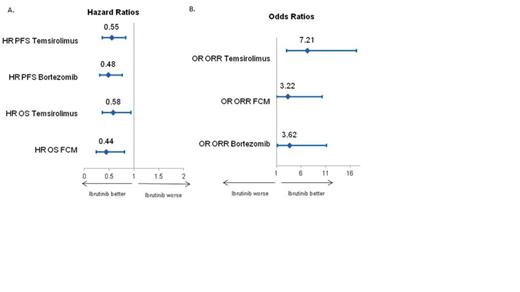
A systematic literature review (SLR) was conducted to identify available clinical trial data for key therapies for treatment of r/r MCL. ORR, PFS, and overall survival (OS) were extracted and patient populations compared. A matching-adjusted indirect comparison (MAIC) of PCYC-1104 versus comparator trials was conducted, adjusting for reported patient population differences. A health state model was then developed to simulate long-term PFS and OS, comparing other therapies with ibrutinib. The model simulated treatment of a cohort of patients with r/r MCL until death or until disease progression, at which point they were simulated to receive subsequent treatment or best supportive care. PCYC-1104 data informed clinical inputs for ibrutinib; hazard ratios (HRs) (PFS and OS) and odds ratios (OR) (response rates) estimated from MAIC were used to inform the effectiveness of the comparators (Figure). Results
Few relevant clinical trials were identified as part of the SLR, with only one randomized Phase 2 study. No Phase 3 trials were identified in the r/r setting. Significant heterogeneity was observed between the study patient populations and time periods when studies were conducted, making it challenging to compare outcomes across trials. Notably, different trial populations had been exposed to different numbers of prior lines of therapy, which has important ramifications for treatment outcomes and therefore modeling comparisons. The MAIC attempted to adjust for reported patient characteristics (Table 1). Notably, the bortezomib patient population differed enough from ibrutinib's that the matching was based on a small number (47) of patients. The resulting OS was not consistent with the reported PFS and response findings due to the higher degree of previous treatments in ibrutinib trial. Due to these data limitations, the simulation focused on comparison of ibrutinib with temsirolimus and fludarabine, cyclophosphamide, and mitoxantrone (FCM) using the results of the MAIC. Simulation over a lifetime horizon resulted in prolonged estimated mean PFS and OS for ibrutinib compared to temsirolimus and FCM. Estimated mean PFS and OS for ibrutinib are 18.4 months and 25.8 months, compared to a 9.6 months and 16.2 months for temsirolimus. Compared with FCM, ibrutinib's mean survival was projected to be over 13 months longer. Conclusion
The paucity of comparative data and the heterogeneity across clinical trial populations make comparing outcomes for r/r MCL treatments challenging. An ongoing Phase III comparative trial of ibrutinib will better inform comparisons with temsirolimus, as MAIC cannot fully adjust for population differences. Based on this simulation model, in which trials were selected for comparability and matching-adjusted to account for differences in patient populations, ibrutinib was projected to prolong PFS and OS in this indication of great unmet need.
Studies Included in MAIC
| Author (Year) . | Treatment . | N of patients in the trial . | N of patients in 1104 trial used for matching* . | Characteristics matched . |
|---|---|---|---|---|
| Fisher (2006) | Bortezomib | 115 | 47 | Age, gender, % refractory, LDH, bone marrow involvement |
| Forstpointner (2004) | FCM | 24 | 111 | Age, gender, number of prior treatments, bone marrow involvement |
| Hess (2009) | Temsirolimus | 54 | 89 | Age, gender, number of prior treatments, prior bortezomib use, bone marrow involvement |
| Author (Year) . | Treatment . | N of patients in the trial . | N of patients in 1104 trial used for matching* . | Characteristics matched . |
|---|---|---|---|---|
| Fisher (2006) | Bortezomib | 115 | 47 | Age, gender, % refractory, LDH, bone marrow involvement |
| Forstpointner (2004) | FCM | 24 | 111 | Age, gender, number of prior treatments, bone marrow involvement |
| Hess (2009) | Temsirolimus | 54 | 89 | Age, gender, number of prior treatments, prior bortezomib use, bone marrow involvement |
*The total number of patients in 1104 trial is 111
Peng:Janssen: Consultancy; Evidera: Employment. Pan:Janssen: Consultancy; Evidera: Employment. Sorensen:Evidera: Employment; Janssen: Consultancy. Dorman:Janssen: Consultancy; Evidera: Employment. Xu:Evidera: Employment; Janssen: Consultancy. Sallum:Evidera: Employment; Janssen: Consultancy. Gaudig:Janssen: Employment. Sengupta:Janssen: Employment. Wildgust:Janssen Global Services: Employment. Sun:Janssen: Employment.
Author notes
Asterisk with author names denotes non-ASH members.