Abstract
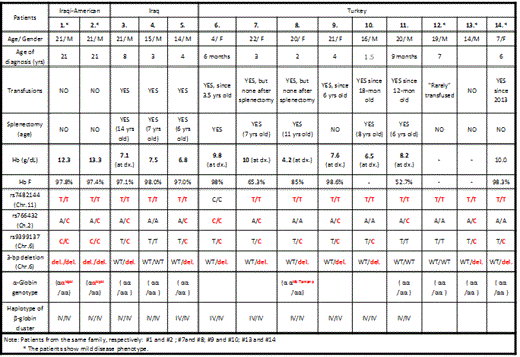
In β-thalassemia major, an inherited disorder caused by homozygosity or compound heterozygosity for β-thalassemia mutations, most patients are severely anemic and require chronic RBC transfusions. Elevated levels of HbF can ameliorate the disease severity and 3 major HbF quantitative trait loci (QTL) on chr 11p, 6q (the HBS1L-MYB intergenic polymorphism) and 2p (BCL11A) are associated with HbF levels in all populations studied. We studied Iraqi-American non-identical twins with markedly elevated HbF who are homozygous for codon 8 (–AA) β0-thalassemia mutation, but are clinically well and transfusion independent. MLPA analysis of the β-globin gene cluster did not show any hereditary persistence of fetal hemoglobin deletions or γ-globin gene quadruplication. Sequencing HBG2 and HBG1 promoters excluded other γ-globin gene promoter non-deletional HPFH mutations. We characterized 12 Turkish and Iraqi patients who are homozygous for codon 8 (–AA) frame-shift β-thalassemia mutation. Among this group, three patients from two Turkish families have a mild disease phenotype, six patients from four Turkish families have a severe phenotype, and three Iraqi patients from three families have a severe phenotype. To assess the contribution of 3 major HbF QTLs to disease severity, we genotyped polymorphisms in these QTL in all 14 patients (Table). Homozygosity for rs7482144 minor alleles (the -158 5' HBG2 Xmn1 C-T SNP) in the HBB gene cluster is present in 13 of 14 patients with the exception being one patient with a severe phenotype. No distinct pattern for the BCL11A sentinel SNP rs766432 minor allele is found between mild and severely affected patients. Homozygosity for rs9399137 minor alleles marking the HBS1L-MYB intergenic region is present in two patients with a mild phenotype, while heterozygosity for minor alleles and homozygosity for major alleles are present in both mild and severe patients. Although the Iraqi-American twins first studied have the most minor alleles (5 of 6 possible minor alleles), the other three patients with mild phenotype have 4, 3 and 2 minor alleles, respectively. Strikingly, one patient with mild phenotype is only homozygous for the rs7482144 minor alleles. Patients with severe phenotypes have 2 to 4 QTL minor alleles. Neither individual QTL genotypes nor the number of QTL minor alleles can distinguish the mild phenotype and severe phenotype in patients with β-thalassemia major of Iraqi and Turkish origin suggesting that other factors contribute to the mild phenotype and high HbF levels in these patients. The entire 14.7 kb intergenic region of between HBG1 and HBD of one twin was sequenced and revealed 19 known SNPs and 4 novel SNPs. The potential roles of these SNPs in regulating γ-globin gene expression are under investigation. We are also investigating whether there are any other potential cis- or trans-acting factors that contribute to mild disease phenotype in these patients using genome-wide SNP arrays, whole genome sequencing and whole exome sequencing. By studying these unusual cases we hope to provide new understanding and insights for elevated HbF production in β-hemoglobinopathies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.