Abstract
Rationale
Patients with sickle cell disease (SCD) are marked by chronic inflammation but the cause of this inflammation and the relevance to patient survival are unknown.
Objective
To assess the relationship between iron, inflammation and early death in SCD.
Methods and Results
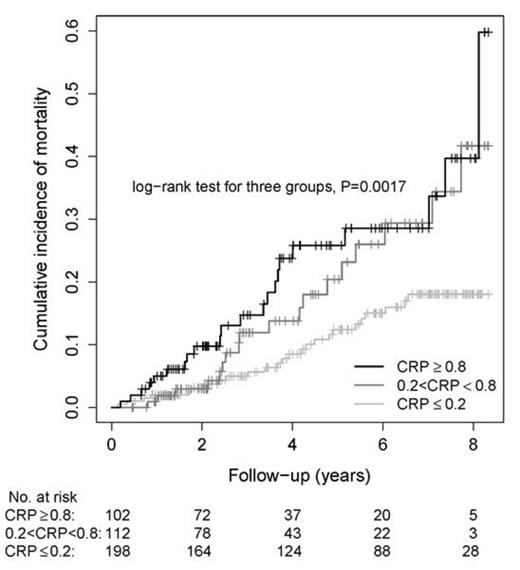
Using peripheral blood mononuclear cell transcriptome profile hierarchical clustering, we classified 24 patients and 10 controls in clusters with significantly different expression of genes known to be regulated by iron. Subsequent gene set enrichment analysis showed that many genes associated with the high iron cluster were involved in innate immunity pattern recognition receptor (PRR) pathways. These included Toll-like receptors TLR4, TLR7 and TLR8; NOD-like receptors NLRP3 and NLRC4; C-type lectin receptor CLEC7A/Dectin-1; humoral pattern recognition receptor pentraxin-3, the common inflammasome effector caspase-1 and one of its substrates, IL-18. Quantitative PCR confirmed quantitative expression of several representative genes and showed that ferritin light chain, TLR4 and interleukin-6 mRNAs were expressed over 100-fold more highly in SCD patients than in controls (P<0.001). In a Mendelian randomization experiment, 14 patients with ferroportin Q248H variant that causes intracellular iron accumulation had significantly higher levels of interleukin-6 and C-reactive protein (CRP) compared to 14 patients with the wild type allele, implying a causal effect (P<0.05). Finally, level of CRP, also a humoral pattern recognition receptor, predicted mortality in the NIH sickle cell cohort (n=412, ClinicalTrials.gov identifier: NCT00011648). Patients in the highest quartile (CRP>0.8 mg/dL) had the highest mortality, and those with the lowest level (CRP<0.2mg/dL, lower limit of detection) had the lowest mortality (P=0.0017, median follow-up 47 months, IQR 24-82, range 2-132)(figure). CRP was independently associated with mortality in a Cox proportional hazards regression model after adjustment for other covariates previously published as associated with mortality (HR 2.8, 95%CI 1.03-4.2, P<0.001, adjusted for white blood cell count, age, transferrin, NT-proBNP and elevated tricuspid regurgitant flow velocity).
Conclusions
The SCD PBMC transcriptome reflects high intracellular iron exposure, which is associated with inflammation and mortality. The associations found in this study support a hypothetical model in which high intracellular iron in macrophages promotes clinically significant inflammatory pathways involving pattern recognition receptors and innate immune function, identifying potential targets for pharmacological intervention with drugs already approved for inflammasome-mediated disorders.
Figure. Kaplan Meier curve showing a significant difference in survival between patients with low and high CRP. SCD Patients (n=412) of the NIH pulmonary hypertension screening cohort (ClinicalTrials.gov identifier: NCT00011648) Patients were divided in high, intermediate and low CRP groups. Kaplan-Meier survival curves show that mortality rate significantly increased according to CRP group.(P=0.0017).Five year mortality percentages for the low, intermediate and high CRP groups were respectively 12.4%, 20.5 and 25.8%. In a multivariate analysis, CRP was an independent predictor of mortality.
van Beers:Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.