Abstract
Introduction:
Langerhans cell histiocytosis (LCH) is a rare proliferative disorder of epidermal antigen-presenting cells such as dendritic cells (DCs). Historically, three distinct clinical syndromes have been described: eosinophilic granuloma (EG), Hand-Schuller-Christian disease and Letterer-Siwe disease (LSD). The disease has a broad spectrum of clinical behavior, from a mild self-limited form like EG-type LCH to an aggressive form associated with a high mortality rate like LSD-type LCH. Since one of the lethal complications of LCH is hemophagocytic syndrome (HPS), investigating a novel treatment approach for LCH associated HPS (LCH-HPS) is of paramount importance.
We have reported the usefulness of cytokine profiles to differentiate HPS from various inflammatory diseases, in which macrophages/monocytes derived cytokines such as interleukin (IL)-18 and neopterin were highly elevated. Since clinical trials using neutralizing antibody to IL-18 in rheumatoid arthritis or plaque psoriasis are now underway, in this study, we examined cytokine profiles over time from six LCH cases and investigated the significance of IL-18 in the pathophysiology of HPS to establish a unique treatment strategy for LCH-HPS.
Materials and Methods:
The concentrations of serum IL-18 were measured in six patients with LCH, including three patients with EG-type LCH and three with LSD-type LCH. Diagnosis of LCH and HPS were based on clinical and histopathological findings. Serum IL-18 levels were measured by enzyme-linked immunosorbent assay (ELISA). Total RNA was extracted from tumor samples for analysis of IL-18 and LANGERIN mRNA expression. BRAF gene mutation was analyzed using DNA extracted from tumor cells as previously described.
Results:
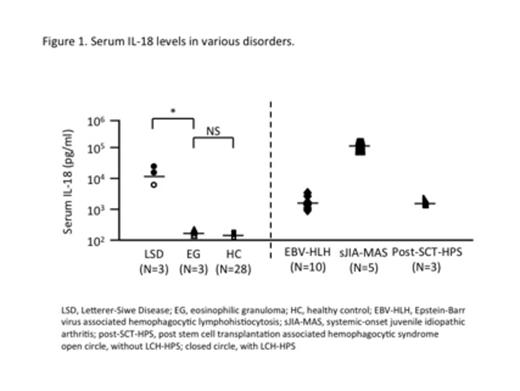
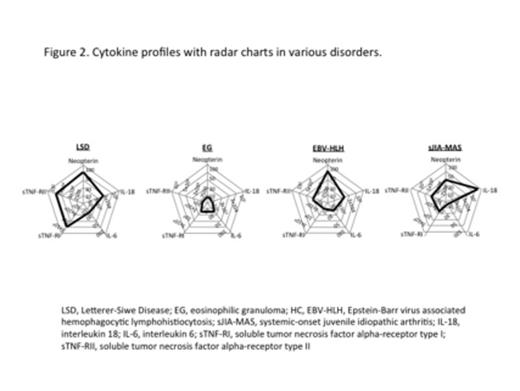
Serum IL-18 level of LSD-type LCH was significantly higher than that of EG-type LCH (median 13,600 vs. 449, p<0.05) (Figure 1). In fact, two out of three LSD-type LCH cases developed LCH-HPS, whereas none of the cases with low level of IL-18 developed. When developed to HPS caused by various disorders, serum IL-18 concentrations became markedly increased as shown in Figure 1, suggesting that IL-18 plays a pivotal role in macrophage activation to develop HPS even in LCH. Serum concentration of IL-18 recovered to and maintained normal range after induction chemotherapy. We also observed a dominant increase of soluble tumor necrosis factor alpha-receptor type I (sTNF-RI) and type II (sTNF-RII) in LSD-LCH cases in addition to essential parameters for HPS: IL-18 and neopterin (Figure 2). This unique cytokine profile of LCH-HPS might be the cause of higher mortality rate (20%) compared to HPS caused by various disorders (5-10%).
BRAF V600E mutation was detected in one of the three cases in each EG-type LCH and LSD-type LCH, suggesting that existence of BRAF V600E mutation does not correlate to the disease severity as previously reported.
As recent studies demonstrated that LCH was originated from DCs, which were close to macrophages/monocytes systems, we hypothesized that LSD-type LCH tumor cells secret IL-18, and then directly initiate macrophage activation to develop HPS. To test this, we assessed IL-18 mRNA expression in tumor cells from LSD-type LCH by RT-PCR. LANGERIN mRNA expression was enhanced from all the LSD-type LCH tumor cells, however IL-18 mRNA did not seem to be derived from tumor cells, suggesting that LSD-type LCH cells themselves would trigger lethal HPS similar to lymphoma associated HPS.
Conclusion:
In conclusion, though LSD-type LCH tumor cells did not express IL-18 directly, the level of IL-18 was much higher in LSD-type LCH. Paying carful attention should be required to the patients with LSD-type LCH with highly elevated IL-18 to prevent the progression of lethal LCH-HPS. To hamper the macrophage activation, an application of IL-18 antibody may be clinically useful for the disease control of LCH-HPS.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract