Abstract
Background: Pediatric severe hemophilia A patients with high titer inhibitors are often reported to have inadequate control of acute bleeding episodes because they do not respond to bypassing agents as predictably as with FVIII therapy for non-inhibitor patients. Thromboelastography (TEG) is a global assay of hemostasis that has promising benefits for use in clinical care for hemophilia patients.
Aims: This study was performed to determine in pediatric inhibitor patients: 1) if baseline TEG, specifically R or reaction time that corresponds to time to thrombin generation and clot formation, is stable over time in individual inhibitor patients; 2) if there are any predictors of baseline TEG R time; 3) to determine response to recombinant activated factor VII (rFVIIa, NovoSeven, NovoNordisk, Copenhagen, Denmark); and 4) to determine predictors of TEG R time following rFVIIa.
Methods: This analysis was conducted within a consented single institution prospective inceptional cohort study. Clinical data regarding healthy volunteers with no personal or family history of a bleeding or clotting disorder and pediatric hemophilia patients with and without inhibitors (assayed by Nijmegen modification of the Bethesda assay (BU)) were extracted from research records and electronic medical records. For this study, TEGs were performed in kaolin citrated samples with added TPA (final concentration 450 ng/mL). Descriptive data were presented as mean and SD. This report analyzed TEG R times, indicating time to clot formation and initial thrombin generation. Baseline TEGs were obtained at least five half-lives after the last infusion of each clotting factor or bypassing agent received. Post treatment TEGs were performed on patients 1 hour following a therapeutic treatment with rFVIIa.
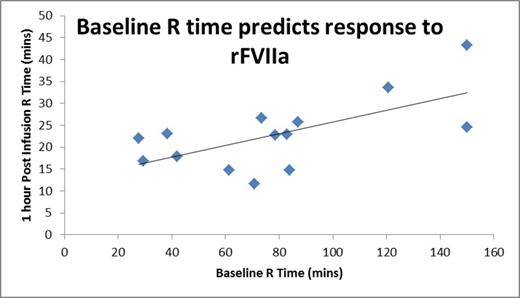
Results: R times on TEGs were obtained on 24 healthy adults, 23 healthy children, 15 children with severe hemophilia A without an inhibitor, and 12 children with severe hemophilia A and an inhibitor. 32 samples were obtained in the 12 children with inhibitors, with 1 to 11 samples from each child. Paired samples were obtained at baseline prior to and 1 hour following a dose (90-270 mg/kg) of rFVIIa. Mean TEG R times were 8.3 minutes (SD 1.4) for healthy adults, 7.7 minutes (SD 1.5) for healthy children, 20.2 minutes (SD 9.4) for children with severe hemophilia A and 102.1 minutes (SD 44.4) for children with severe hemophilia A and inhibitors. Healthy children did not differ from healthy adults (p=0.43), but children with hemophilia with inhibitors differed from healthy controls (p=0.046) and trended toward differences from children with hemophilia without inhibitors (p=0.08, however limited in sample number). Baseline R values in children with inhibitors did not correlate with age (r=-0.19) or inhibitor titer (r=0.23). Children studied on multiple occasions showed variability over time. TEG 1 hour following rFVIIa in a baseline state showed a mean R time of 25.1 minutes (SD 7.1). Post rFVIIa R time did not correlate with age (r=0.21) or inhibitor titer (r=-0.14), but showed considerable correlation with baseline TEG R time (Figure 1, r =0.65, p=0.013). Following infusion of rFVIIa, TEG R times of children with inhibitors never achieved the normal range. However, when rFVIIa was studied following multiple infusions without a washout, the mean TEG R was moderately, but non-significantly shorter at 20.6 minutes (SD 5.6).
Conclusions: TEG R times in young children with severe hemophilia A with inhibitors are greatly prolonged compared to healthy children or adults, and moderately longer than that in children with severe hemophilia A without inhibitors. Baseline TEG R varies over time and cannot be predicted by age or inhibitor titer. Baseline TEG R time may be an important predictor of response to bypassing therapy and serial monitoring over time may be clinically useful to guide therapy. During the course of multiple infusions, moderately improved TEG R responses were determined compared with first infusions. This may, in part, explain our previously reported observation of longer duration of rFVIIa dosing in young children with inhibitors. Future studies employing TEG to help optimize response to bypassing agents are needed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract