Abstract
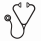
Background and Objective: No-VKA oral anticoagulants (NOAC) have been approved for acute and extended treatment of venous thromboembolism (VTE) or long-term anticoagulation in atrial fibrillation (AF). A major concern of physicians is the fear of uncontrolled bleeding or cardiovascular events during NOAC therapy resulting in fatal outcomes. We evaluated causes of death in a large cohort of NOAC registry patients.
Methods: The prospective NOAC registry was initiated in November 2011. A network of more than 230 physicians in the district of Saxony, Germany, enrol up to 3000 patients in the registry, which are prospectively followed by the central registry office for up to 36 months. Every death was centrally adjudicated and categorized according to standard definitions.
Results: Until June 30th 2014, 2667 patients were enrolled into the registry. Of these, 1818 (68.2%) patients received rivaroxaban, 348 (13.0%) received dabigatran and 501 (18.8%) received apixaban. NOAC indication was atrial fibrillation (AF) in 2025 (75.9%) cases, venous thromboembolism (VTE) in 609 (22.8%), and other indications in 33 (1.2%) cases. Patients had a mean age of 71.9 years (range 14–100y) and 1415 (53.1%) patients were male.
At present, 18396 completed FU correlate to 9087.9 patient years. During follow-up, 173 patients died (1.9 per 100 patient years). Causes of death are presented in table 1.
Cardiovascular death was the most common cause of death but mainly consisted of sudden cardiac death and chronic heart failure and rarely consisted of acute thromboembolic events. Fatal bleeding accounted for 7.5% of all fatalities. More than 34% of deaths could be attributed to acute infections or terminal malignant disease.
Conclusion: In patients receiving NOAC therapy, mortality mainly relates to age, sudden cardiac death, malignant disease or acute infections. Thromboembolic events and fatal bleeding together account for only 13.2% of all fatalities, indicating the road therapeutic window for NOACs even in terminally ill patients.
causes of death in all patients and in actively anticoagulated patients in the Dresden NOAC registry
| Cause of death . | All deaths n=173 . | deaths during anticoagulant treatment (NOAC and other anticoagulation) n= 125 . | |
|---|---|---|---|
| fatal cardiovascular event | sudden cardiac death | 32/173 (18.5) | 22/125 (17.6) |
| stroke | 4/173 (2.3) | 3/125 (2.4) | |
| ACS | 3/173 (1.7) | 2/125 (1.6) | |
| VTE | 3/173 (1.7) | 2/125 (1.6) | |
| Other fatal CV (e.g. chronic heart failure) | 31/173 (17.9) | 23/125 (18.4) | |
| fatal bleed | 13/173 (7.5) | 12/125 (9.6) | |
| terminal malignant disease | 29/173 (16.8) | 20/125 (16.0) | |
| age related death | 19/173 (11.0) | 12/125 (9.6) | |
| infection/sepsis | 31/173 (17.9) | 22/125 (17.6) | |
| other | 8/173 (4.6) | 7/125 (5.6) | |
| Cause of death . | All deaths n=173 . | deaths during anticoagulant treatment (NOAC and other anticoagulation) n= 125 . | |
|---|---|---|---|
| fatal cardiovascular event | sudden cardiac death | 32/173 (18.5) | 22/125 (17.6) |
| stroke | 4/173 (2.3) | 3/125 (2.4) | |
| ACS | 3/173 (1.7) | 2/125 (1.6) | |
| VTE | 3/173 (1.7) | 2/125 (1.6) | |
| Other fatal CV (e.g. chronic heart failure) | 31/173 (17.9) | 23/125 (18.4) | |
| fatal bleed | 13/173 (7.5) | 12/125 (9.6) | |
| terminal malignant disease | 29/173 (16.8) | 20/125 (16.0) | |
| age related death | 19/173 (11.0) | 12/125 (9.6) | |
| infection/sepsis | 31/173 (17.9) | 22/125 (17.6) | |
| other | 8/173 (4.6) | 7/125 (5.6) | |
causes of death in all patients (1a) and more detailed analysis of the type of cardiovascular death (1b)
causes of death in all patients (1a) and more detailed analysis of the type of cardiovascular death (1b)
Werth:Bayer: Honoraria. Köhler:Bayer: Honoraria. Beyer-Westendorf:Boehringer Ingelheim: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Bayer: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract