Abstract
Introduction. Ultra-deep next generation sequencing (NGS) allows sensitive detection of mutations and estimation of their clonal abundance in tumor cell populations. TP53 mutations identified by ultra-deep NGS significantly impact on survival of chronic lymphocytic leukemia (CLL) patients, independent of their representation in the tumor clone and even if restricted to a small fraction of leukemic cells.
Purpose. Here we aim at assessing the frequency, prognostic impact and evolution during disease course of NOTCH1, SF3B1 and BIRC3 mutations identified by ultra-deep NGS in newly diagnosed CLL.
Methods. The study was based on a consecutive series of 304 newly diagnosed and previously untreated CLL (median age: 71 years; Binet A/B/C: 79/12/9%; unmutated IGHV genes: 35%; del13q/+12/del11q/del17p: 51/21/8/9%; median follow-up: 8.1 years). By ultra-deep NGS, TP53 mutations (exons 4-8) occurred in 15% of CLL with a median clonal abundance of 18% (range 0.2-95%). NOTCH1 (hg19 g.chr9:139390596-139390829), SF3B1 (exons 14, 15) and BIRC3 (exons 6, 9) mutations were screened on peripheral blood samples by amplicon-based ultra-deep NGS (454 Life Sciences) (average depth: 2437). A bioinformatic algorithm was applied to call non-silent variants out of background NGS noise. Variant calling by the algorithm was validated by Sanger sequencing or, if the variant was below the sensitivity threshold of Sanger sequencing, by both duplicate ultra-deep NGS and allele specific PCR (AS-PCR). Variant allele frequency (VAF) was corrected for tumor representation.
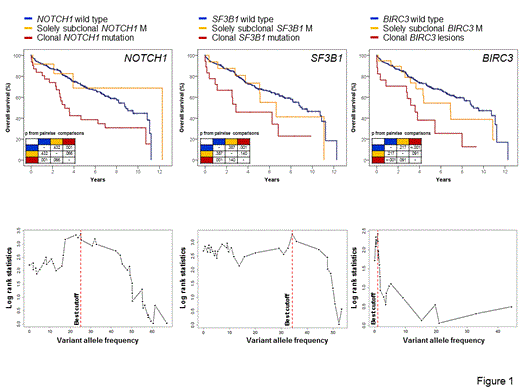
Results. Ultra-deep NGS identified 46 NOTCH1 mutations (median VAF 24%; range: 1.4-64%) in 14% (43/304) CLL, 43 SF3B1 mutations (median VAF 16%; range: 0.5-48%) in 11% (35/304) CLL and 37 BIRC3 mutations (median VAF 5.6%; range: 0.2-47%) in 8% (26/304) CLL. In cases harboring more than one mutation (NOTCH1=3; SF3B1=2; BIRC3=5), the variants mapped on distinct sequencing reads from the same amplicon suggesting that they belonged to different CLL subclones. Out of the variants identified by ultra-deep NGS, a significant fraction of NOTCH1 (n=14; median VAF: 3.9%; range: 1.4-9%), SF3B1 (n=25; median VAF: 4.3%; range: 0.5-17%) and BIRC3 (n=30; median VAF: 1.4%; range: 0.2-6%) mutations were missed by Sanger sequencing because their VAF was below the sensitivity threshold of the assay (~10%). The molecular spectrum of these small subclonal mutations was highly consistent with that of mutations that were visible by Sanger sequencing. The overall survival of cases harboring solely small subclonal mutations of NOTCH1, SF3B1 and BIRC3 was similar to that of wild type cases and longer than that of cases with clonal lesions (Fig. 1). Outcome-driven statistical approaches (maxstat and recursive partitioning) were applied to identify the optimal cut-off of the VAF in order to test whether the clonal representation of mutations correlated with CLL survival. Application of these approaches to TP53 mutations failed in identifying a clear cut-off, suggesting that TP53 variants are relevant to the outcome of CLL independent of their VAF. Conversely, in the case of NOTCH1, SF3B1 and BIRC3 mutations, these approaches consistently identified 25%, 35% and 1%, respectively, as the best VAF cut-off values for outcome prediction (Fig. 1), thus suggesting that small subclones harboring NOTCH1, SF3B1 or BIRC3 mutations are clinically irrelevant. By multivariate analysis, NOTCH1 mutations >25% of VAF (HR: 1.8; p=.038), SF3B1 mutations >35% of VAF (HR: 2.9; p=.005) and BIRC3 mutations >1% of VAF (HR: 1.8; p=.044) were significantly associated with an increase in the hazard of death, after adjusting for TP53 lesions (HR: 2.9; p<.001), del11q (HR: 2.2; p=.007), +12 (HR: 1.3, p=.174) and del13q (HR: 0.8; p=.512). Among cases harboring small mutated subclones, longitudinal deep NGS of sequential samples documented the outgrowth of the variants to a clonal level in 1/5 cases for NOTCH1, in 5/12 for SF3B1 and in 3/10 for BIRC3. While selection of NOTCH1 and BIRC3 variants did not associate neither with disease feature nor with treatment, the outgrowth of subclonal SF3B1 mutations was restricted to cases showing unmutated IGHV genes (p=.015).
Conclusions. Small mutated subclones of the NOTCH1, SF3B1 and BIRC3 genes appear to be clinically irrelevant. Mutations of NOTCH1 and SF3B1 require a substantial clonal representation to be harmful.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.