Abstract
INTRODUCTION: Prior to the introduction of bosutinib and ponatinib, CP-CML patients who had failed 2 prior lines of TKI had limited treatment options with generally poor treatment response and outcomes. These newer TKIs are important additions to the treatment armamentarium, but the optimal choice of 3rd-line (3L) treatment has not been established. Clinical trials in patients who have failed 2 previous lines of TKI suggest ponatinib is more efficacious than bosutinib, but with a less favorable side-effect profile, requiring a potential benefit-risk tradeoff in treatment choice. Comparison of overall benefit-risk from available clinical trial data is challenging due to single- arm designs, low overall mortality, disparate impact of characteristic side-effects, and the likely crossover/sequential use of alternative TKIs among patients discontinuing therapy. We therefore examined efficacy outcomes previously validated as predictors of long-term survival, as well as treatment duration and reason for study drug discontinuation, as surrogates for overall benefit-risk in 3L CP-CML patients treated with ponatinib vs. bosutinib.
METHODS: Using clinical trial data for 3L CP-CML from Khoury 2012 for bosutinib and PACE (unpublished) for ponatinib, we examined efficacy outcomes including major (MCyR) and complete (CCyR) cytogenetic response, and major molecular response (MMR), durability of response, duration on therapy and reasons for discontinuation among patients treated with ponatinib vs. bosutinib after failing 2 prior TKIs. Outcomes were evaluated at similar follow-up time points: median 28.5 (range 0.3-56.2) months bosutinib; median 30.5 (0.2-39.8) months ponatinib. Efficacy outcomes were defined such that patients were required to demonstrate improvement relative to baseline to be counted as responders. Investigator-reported primary reason for discontinuation was examined and considered caused by treatment failure if due to death, disease progression or unsatisfactory response.
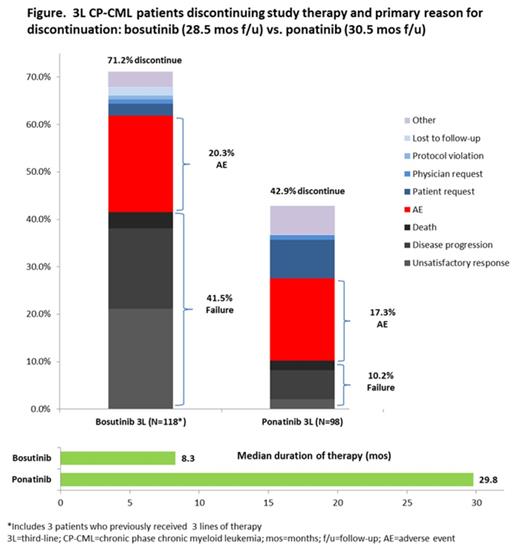
RESULTS: Treatment response was higher for 3L CP-CML patients treated with ponatinib (N=98) than with bosutinib (N=118) across all measures. MCyR was achieved by 67% of ponatinib vs. 32% of bosutinib patients, CCyR by 56% of ponatinib vs. 24% of bosutinib patients, and MMR by 42% vs. 15%. Patients who received ponatinib experienced more durable responses with 93% of the ponatinib patients who achieved MCyR estimated to retain response after 2 years vs. 59% of the bosutinib patients who achieved this response level. After approximately 2.5 years of follow up, less than one-third (29%) of bosutinib patients remained on study drug vs. the majority (57%) of ponatinib patients (Figure). The median treatment duration was substantially shorter for bosutinib vs. ponatinib, with patients remaining on ponatinib therapy more than 3.5 times as long as on bosutinib (Figure). The majority of 3L bosutinib patients that discontinued did so due to treatment failure (58.3% of the patients who discontinued), while less than one-quarter (23.8%) of ponatinib patients who discontinued did so due to failure. The impact of AEs on discontinuation was similar: about 1 in 5 patients on both therapies discontinued due to AEs.
CONCLUSIONS: Our indirect comparison using a variety of surrogate measures provides strong evidence of superior efficacy and durability of response with ponatinib vs. bosutinib in 3L CP-CML patients; the higher proportion of ponatinib patients continuing on therapy and the longer duration of therapy also suggest patients experience better overall response and tolerability outcomes with ponatinib vs. bosutinib. Based on the surrogate measures of patient benefit- risk examined in this analysis, ponatinib appears to provide a net overall benefit vs. bosutinib in 3L CP-CML patients.
Levy:ARIAD Pharmaceuticals, Inc.: Consultancy, This ASH abstract publication value, advisory board speaking engagement, advisory board participation Other. McGarry:ARIAD Pharmaceuticals, Inc.: Employment, Equity Ownership. Huang:ARIAD Pharmaceuticals, Inc.: Employment, Equity Ownership. Lustgarten:ARIAD Pharmaceuticals Inc: Employment, Equity Ownership. Nieset:ARIAD Pharmaceuticals, Inc.: Employment, Equity Ownership. Haluska:ARIAD Pharmaceuticals, Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.