Abstract
Immunologic tolerance is a critical homeostatic function to protect self from auto-immunity. Various immune-regulatory cells, including regulatory T cell (Treg), myeloid derived suppressor cell (MDSC), tolerogenic dendritic cell, and mesenchymal stromal cell (MSC) are responsible for orchestrating this tolerance. Immune-regulatory cells play a central role in the pathophysiology of cancer immunity, autoimmune disease, and graft versus host disease and can be used in adoptive cell therapy. There is therefore a need for a standardized method to evaluate the suppressive function of these heterogeneous cell populations. We developed an in vitro standardized quantitative suppression assay utilizing the suppressor cell line KARPAS-299 as a standard by which to compare suppressive potency of human immune-regulatory cell populations.
Peripheral CD4 T-cells, used as targets in all assays, were isolated from healthy donors (n=30) using an automated cell separator or by flow sorting. After labeling with Cell Tracer Violet (CTV), CD4 T-cells were co-cultured with immune-regulatory cells such as autologous Treg, autologous or third party MDSC (CD11b+CD14+), third party bone marrow derived MSC, and primary leukemia cells, or with the reference KARPAS-299 cells. After stimulating with CD3/CD28 beads, CD154 activation of CD4 T-cells was measured at 16 hours, and CD4 T-cell proliferation was measured by CTV dilution within the viable cell population at 72 hours. Suppressive capacities of immune-regulatory cell types were represented as KARPAS-299 suppressor units (KSU), calculated using the equation b/a, where b is the percentage of suppression in the presence of a given immune-regulatory cell, and a is the percentage of suppression in the presence of KARPAS-299.
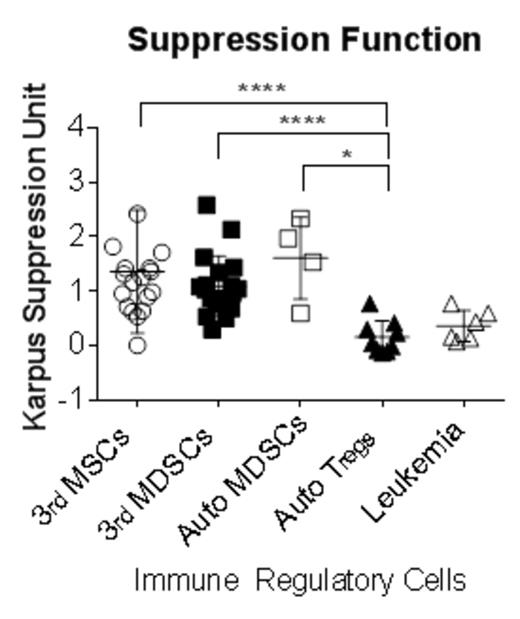
KARPAS-299 cells reproducibly suppressed healthy donor CD4 T-cells at suppressor: responder ratio of 4:1 for both CD154 activation (percentage of suppression 46.2±18.8%) and proliferation (51.0±20.7%). Immune-regulatory cells showed diverse suppressive capacities: autologous MDSC (63.2±24.5%), allogeneic MDSC (55.8±21.8%), third party MSC (51.26±23.67%), and autologous Treg, (8.7±10.4%). After standardization with KSU, MDSC and MSC showed significantly higher suppressive capacity compared to Treg (MDSC 1.18±0.6 KSU, MSC 1.3±1.11 KSU, Treg 0.15±0.3 KSU: p<0.0001). This standardized assay was also applicable to other types of proliferating targets, such as flow-sorted conventional T cells (Tcon: CD4+CD127highCD25low: 2.73±1.78 KSU with autologous MDSC, 1.6±0.75 KSU with third party MSC) and CD8 T-cells (1.49±0.96 KSU with autologous MDSC, 1.08±0.77 with third party MSC). We validated the method as a potency assay of MSC products, and showed inter-individual differences in MSC. Finally, we used the assay to demonstrate a modest suppressive capacity of acute myeloid leukemia blasts (0.35±0.28 KSU).
This method provides a platform for standardizing suppressor function to facilitate comparison between laboratories and for use in cell product release assay.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.