Abstract
SF3B1 is a critical component of the RNA splicing machinery that achieves successful transcription and provides the functional diversity of protein species through alternative splicing. Recent studies have identified SF3B1 mutations in several tumours. In chronic lymphocytic leukemia (CLL) these mutations are associated with altered splicing, reduced survival and resistance to treatment. We therefore hypothesised that inhibition of the spliceosome may be an effective therapeutic strategy in SF3B1 mutated individuals.
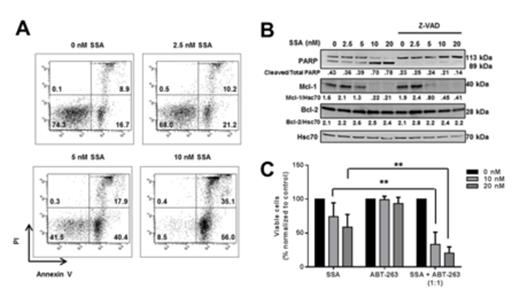
Initially, we utilized Spliceostatin A (SSA), an agent known to inhibit SF3B1 and alter gene splicing in various cell types. We showed that in CLL cells (SF3B1 WT and mutated samples), SSA treatment resulted in a dose-dependent increase in the un-spliced mRNA of DNAJB1 and RIOK3, two genes known to be regulated by SF3B1. We then assessed the ability of SSA to kill CLL cells with an SF3B1 mutant (n=12) or wild-type (n=28) genotype over a 24 hour period. CLL cells, regardless of SF3B1 mutational status, underwent apoptosis in a dose- (Figure A) and time-dependent manner (data not shown), with a mean IC50 of 5.1 nM. Interestingly, SSA induced more apoptosis in IGHV unmutated-CLL (U-CLL) compared to mutated-CLL samples (M-CLL) (p=0.037) and was active in 17p-deleted CLL (n=7), an aggressive disease sub-type that exhibits pronounced resistance to standard treatments. Normal B and T cells were significantly more resistant to SSA treatment than CLL samples purified from age-matched normal donors (B-cells p=0.006, T-cells p=0.001) and CLL derived T cells (p=0.003).
SSA-induced apoptosis proceeded via a caspase-dependent mechanism (inhibited by ZVAD.FMK) coincident with elevations in Noxa. No changes in Bcl-2 or Bcl-xL were observed. Given the known ability of Noxa to interact with, and destabilise, Mcl-1, we next assessed the ability of SSA to modulate Mcl-1, and observed a substantial decrease in protein expression (Figure B, p=0.028). SSA treatment also altered the splicing of MCL-1; reducing the proportion of full length MCL-1L and increasing the amount of MCL-1s transcripts produced. The key role of Mcl-1 in regulating SSA-induced apoptosis was also observed in Ramos cells where over-expression of Mcl-1L, significantly protected them from SSA-induced apoptosis (p=0.001). Furthermore, in CLL cells exposed to CD40L and IL-4, factors known to induce Mcl-1 expression, SSA-induced apoptosis was significantly reduced (p=0.008). Taken together, these data implicate aberrant Mcl-1 splicing and Noxa upregulation in the apoptosis induced after SSA exposure. CD40L and IL-4 are proposed to be key factors preventing the apoptosis of CLL cells in the proliferative niche. Therefore, to explore possible strategies for overcoming this resistance, we investigated combining SSA with the Bcl-2/Bcl-xL inhibitor ABT-263 and showed that dual exposure to both inhibitors significantly increased apoptosis overcoming the protective effects of IL-4/CD40L (p=0.004; Figure C).
In conclusion, our data shows that SSA induces apoptosis at nanomolar concentrations in CLL cells, independently of SF3B1 mutational status, through effects on Mcl-1. In combination with ABT-263, we show that SSA is particular active and can overcome the protective effects of CD40L and IL-4, suggesting promising clinical utility.
(A) Annexin V /PI analysis of CLL cells treated with SSA, (B) CLL cells treated with SSA for 24 h followed by immunoblotting for various proteins. (C) Drug combination studies with ABT-263 and SSA at a 1:1 ratio at 10 nM or 20 nM following IL-4/CD40L treatment.
(A) Annexin V /PI analysis of CLL cells treated with SSA, (B) CLL cells treated with SSA for 24 h followed by immunoblotting for various proteins. (C) Drug combination studies with ABT-263 and SSA at a 1:1 ratio at 10 nM or 20 nM following IL-4/CD40L treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.