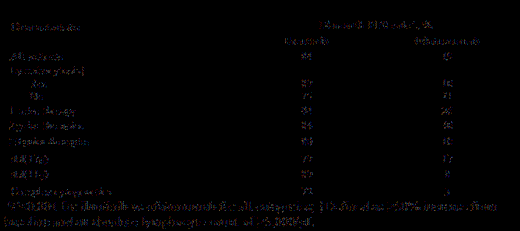Abstract
Introduction: The role of various prognostic factors in CLL/SLL is not yet fully understood, including the implications of new genetic markers associated with high risk. Ibrutinib (Imbruvica®), a first-in-class Bruton’s tyrosine kinase inhibitor, is a once-daily single agent approved by the US FDA for CLL patients (pts) who have received ≥ 1 prior therapy, and for pts with 17p deletion CLL. We report updated efficacy results for the phase 3 RESONATETM(PCYC-1112) study of ibrutinib (ibr) vs ofatumumab (ofa), relative to genetic features and prior treatment exposure, and provide updated adverse event (AE) data.
Methods: Pts received 420 mg oral ibr daily until disease progression or unacceptable toxicity or IV ofa for up to 24 weeks. Primary endpoint was IRC-assessed PFS with secondary endpoints OS and IRC ORR (Byrd et al, NEJM 2014). At interim analysis, with median follow-up of 9.4 months, the Independent Data Monitoring Committee recommended pts in the ofa arm be provided access to ibr. Additional endpoints including investigator-assessed efficacy, investigation of potential predictive biomarkers, and safety with longer follow-up are reported.
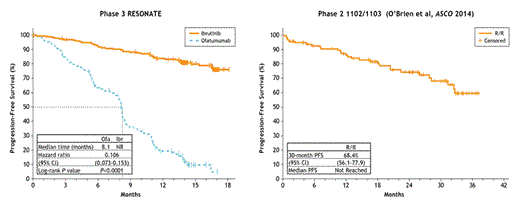
Results: The 391 pts were randomized to ibr (n=195) or ofa (n=196). Median age was 67 years, with 40% ≥70 years, 57% Rai stage III/IV disease. Median number of prior therapies was 3 (ibr) vs 2 (ofa) with 23% having 1, 28% with 2, and 49% ≥ 3 prior therapies. Approximately 32% had del(17p) and 32% (122/381) del(11q). 72 of 300 pts had complex cytogenetics (≥3 abnormalities) and 68% (181/266) unmutated IGHV. With outcome analyses pending, preliminary analysis revealed that 19% of pts had NOTCH1, 23% SF3B1, 36% TP53, and 1% MYD88 gene mutations at baseline. With median follow-up of 16 months, investigator-assessed PFS was significantly longer for ibr vs ofa (median NR vs 8.1 months, [HR 0.106, 95%CI 0.073-0.153, P<0.0001]) (figure). OS was significantly better for ibr vs ofa, with 18-month OS rates of 85% and 78% respectively, despite 120 pts (61%) randomized to ofa who crossed over to ibr and were censored at that time. Higher number of prior therapies (≥3) and 11q deletion were associated with significantly lower 12-month PFS rate for ofa (P=0.001) but not for ibr (P=0.14) and (P=0.13), respectively. The best ORR as assessed by investigator for ibr vs ofa was 90% vs 25% (P<0.0001), including 8% of pts on ibr achieving PR with lymphocytosis and 6% with CR/CRi. Compared with ofa, ibr improved 12-month PFS (table) and ORR regardless of baseline genetics, complex cytogenetics, or number of prior therapies (P<0.001 for ibr vs ofa for all comparisons). Of pts receiving ibr, those who received only 1 prior therapy had better efficacy outcomes than those who received ibr in later lines (12-month PFS of 94% vs 82% [P=0.01]); responses were achieved in 100% vs 88% (P=0.04), of these pts, respectively. No significant difference in 12-month PFS was observed in ibr-treated patients with or without del(17p) or for those who developed lymphocytosis compared to those without lymphocytosis. Median treatment duration was longer for ibr vs ofa (16 vs 5 months). The most common cumulative AEs for ibr, displayed here by maximum grade, were consistent with those reported at interim analysis including diarrhea (37% grade [G]1, 10% G2, 4% G3), fatigue (18% G1, 12% G2, 3% G3), and nausea (24% G1, 6% G2, 2% G3). The most frequent grade 3/4 AEs for ibr included neutropenia (18%), pneumonia (9%), thrombocytopenia (6%), anemia (6%) and hypertension (6%). Atrial fibrillation of any grade occurred in 7% of ibr-treated pts. 47 pts (24%) discontinued ibr; 17 (9%) for progressive disease, 13 (7%) for AEs, 10 (5%) deaths, 3 (2%) pt decision, including 2 pts going to commercial ibr, and 4 (2%) investigator decision, including 1 (1%) for SCT. 76% of pts randomized to ibr continue treatment on study.
Conclusions: Ibr significantly improved investigator-assessed PFS, OS, and ORR relative to ofa in pts with CLL/SLL who had received ≥ 1 prior therapy. The updated results are consistent with previously published phase 2 single-agent results (Byrd et al. NEJM 2013). Pts in the ibr arm treated in the second line experienced better outcomes than those salvaged in later lines of therapy. These results provide further evidence of ibrutinib’s robust clinical activity in CLL pts regardless of high-risk baseline genetics or number of prior therapies.
Brown:Sanofi, Onyx, Vertex, Novartis, Boehringer, GSK, Roche/Genentech, Emergent, Morphosys, Celgene, Janssen, Pharmacyclics, Gilead: Consultancy. Hillmen:Pharmacyclics, Janssen, Gilead, Roche: Honoraria, Research Funding. O'Brien:Amgen, Celgene, GSK: Consultancy; CLL Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; Emergent, Genentech, Gilead, Infinity, Pharmacyclics, Spectrum: Consultancy, Research Funding; MorphoSys, Acerta, TG Therapeutics: Research Funding. Barrientos:Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Coutre:Janssen, Pharmacyclics: Honoraria, Research Funding. Tam:Pharmacyclics and Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Mulligan:Roche, Abbvie: Consultancy, Honoraria. Jaeger:Janssen Cilag: Honoraria. Barr:Pharmacyclics: Research Funding. Furman:Pharmacyclics: Consultancy, Speakers Bureau. Kipps:Pharmacyclics: Research Funding. Cymbalista:Janssen, Roche, GSK, Gilead, Mundipharma: Honoraria. Delgado:Roche: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Speakers Bureau; GSK: Consultancy, Honoraria, Research Funding; Mundipharma: Consultancy, Honoraria; Celgene: Honoraria; Novartis: Consultancy, Honoraria. Montillo:Janssen: Honoraria. Burger:Pharmacyclics: Consultancy, Honoraria, Research Funding. Chung:Pharmacyclics: Employment. Lin:Pharmacyclics: Employment. Gau:Pharmacyclics: Employment. Chang:Pharmacyclics: Employment. McGreivy:Pharmacyclics: Employment. James:Pharmacyclics: Employment. Byrd:Pharmacyclics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract