Abstract
Introduction
Various prognostic factors for multiple myeloma (MM) have been identified, and combinations of these factors were suggested for staging classification of MM patients. The international staging system (ISS) is a widely accepted prognostic system for MM patients. However, since ISS was established using data collected from 1981 through 2002, only a small portion of patients who received the novel agents (e.g., thalidomide, bortezomib, and lenalidomide) were included. Therefore its validity is being challenged in the era of novel agents. We aimed to develop an alternative prognostic index for MM patients based on easily obtained laboratory measures in the era of novel agents.
Patients and methods
Data were collected from February 2001 through June 2012 at Asan Medical Center. 470 patients were newly diagnosed as symptomatic MM and 156 of them were treated with novel agents including thalidomide, lenalidomide and bortezomib. We analyzed these 156 patients who received novel agent therapy at least once, using prospectively collected data. A new prognostic model was developed based on significant potential prognostic factors in univariate and multivariate analysis, which was validated in external validation data set from two tertiary hospitals in Korea.
Results
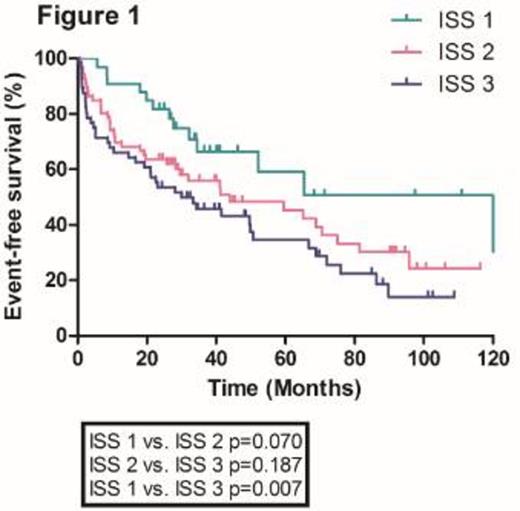
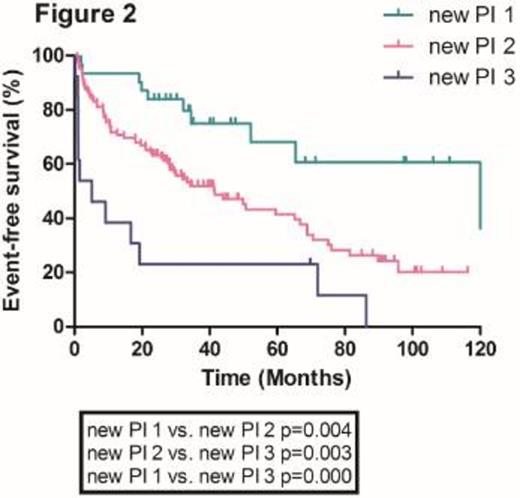
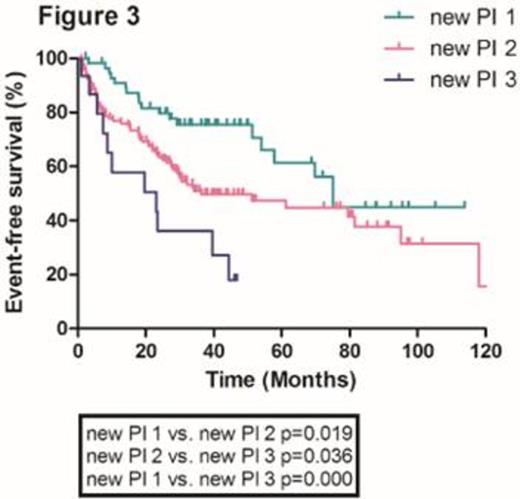
With a median follow-up duration of 27.9 months (range, 0.2-122 months), the median overall survival in the whole population was 28.7 months. The median overall survival (OS) of patients according to ISS stage 1, 2 and 3 were 122.2, 43.9 and 30.0 months, respectively. There were no significant differences in median OS between ISS stage 1 and 2 patients and 2 and 3 patients (Figure 1). Furthermore, ISS was not statistically significant in the multivariate analysis. We incorporated serum beta2-microglobulin (Sβ2M), serum albumin and serum lactate dehydrogenase (LDH) as independent risk factors to develop a new prognostic model. Using these three factors, we stratified the patients into three groups; Group 1, Sβ2M < 5.5 mg/L plus serum albumin >= 3.5 g/dL plus serum LDH normal; Group 2, neither group 1 nor 3; Group 3, Sβ2M >= 5.5 mg/L plus serum albumin < 3.5 g/dL plus serum LDH > normal. We found that there were significant differences in median OS among these three groups (122.2, 41.6 and 5.6 months for group 1, 2 and 3, respectively) (Figure 2). This new prognostic index was further validated in the external cohorts (Median OS was 75.2, 36.3 and 23.1 months for group 1, 2 and 3, respectively) (Figure 3).
Conclusion
Our study suggests that this new prognostic index based on easily obtainable variables (Sβ2M, serum albumin, serum LDH) might be a simple and useful tool to predict prognosis for patients with MM in the era of novel agents, which warrants further validation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.