Abstract
Background: Multiple myeloma is still lethal disease. However, the prognosis of this disease has been improving according to the administration of novel agents. Among of these novel agents, lenalidomide is confirmed the validity of consolidation-maintenance setting by a randomized controlled study. The combination of clarithromycin, lenalidomide and dexamethasone (BiRd) has led to highly durable responses in newly diagnosed myeloma (Rossi A et al 2013). However, mechanism of clarithromycin against myeloma cells is not still clear. It is believed that clarithromycin increases the area under the curve and maximum concentration levels of corticosteroids. On the other hand, clarithromycin has an ability to interact with human MDR1 (ATP-binding Cassette Sub-family B Member 1 (ABCB1), P- glycoprotein). Furthermore, lenalidomide is a substrate of MDR1, a membrane efflux transporter ubiquitously expressed in human tissues, such as the small intestine, whose activity could decrease the bioavailability of lenalidomide. Therefore, we examined whether blood concentration of lenalidomide would change with the existence of clarithromycin.
Aim: To investigate whether blood concentration of lenalidomide would change with the existence of clarithromycin.
Method: Lenalidomide 15 mg (Revlimid; Celgene Corporation, Tokyo, Japan) was orally administered once daily at 08:00 hours according to the recommendations (day1-21) of a 28-day cycle. Dexamethasone (20mg) was administrated on day 1,8,15, and 22. Orally, from day 8 to 21, Clarithromycin 400mg was administrated twice daily. On day 7and 14 of Bird therapy, whole-blood samples were collected just before oral lenalidomide administration, and at 1, 2, 4, and 6 hours thereafter. Pharmacokinetic analysis of lenalidomide was carried out using the standard non-compartmental method using WinNonlin (version 5.2; Pharsight Co, Mountain View, CA). The elimination half-life was calculated from the log-linear regression of the terminal phase of the concentration–time curve using at least 3 sampling points (elimination half-life = ln2/ke; ke = elimination rate constant). The total AUC was calculated using the linear trapezoidal rule.
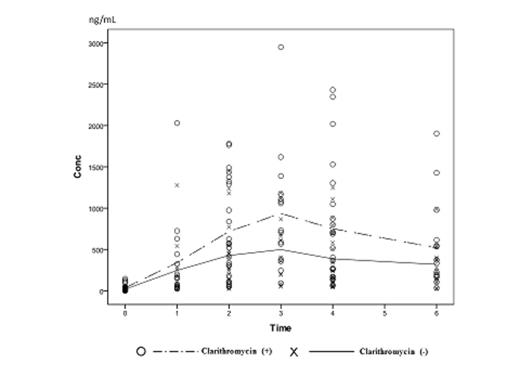
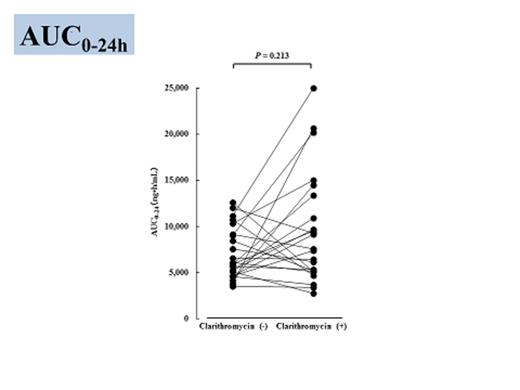
Results: Twenty five patients, who were obtained written informed consent, were enrolled in this study from April 2012 to June 2014. Mean plasma lenalidomide concentrations are shown in Figure 1. According to administration of clarithromycin, plasma concentrations of lenalidomide elevated at 2, 3, and 4 hour, respectably (p=0.045, p=0.039, p=0.042). Furthermore, baseline plasma concentration of lenalidomide was not affected by administration of clarithromycin (p=0.132). On the other hand, AUC24 were not affected by addition of clarithromycin (p=0.213) (Figure 2). In some patients, blood concentration of lenalidomide extremely increased administration of clarithromycin. These patients had wild type of ABCB1, C3435T genotype (C/C) (p=0.036). The other patients who were moderate affected to clarithromycin administration were mutated types (C/T or T/T). Nineteen patients obtained at least VGPR (sCR (9), VGPR (10)). The major adverse event (AE) was skin rush; however, it was manageable, except one patient (Grade 3). Hematological AEs were well tolerable (i.e. Grade 1 or 2, thrombocytopenia). No patient died during BiRd therapy.
Discussion: In MM-001 trial, lenalidomide led anti-MM response according to dose dependent manner (Richardson P, et al. 2002). In addition, hematological AEs, especially thrombocytopenia were significant related to AUC24 (p<0.001). Our trial revealed that administration of clarithromycin led to elevate the maximum concentration of lenalidomide acceding to raising the absorption via inhibition of MDR1. On the other hand, administration of clarithromycin did not affect to the baseline plasma concentration of lenalidomide, so we considered that administration of clarithromycin did not affect to renal excretion. For this reason, if the renal function was sufficient, lenalidomide was excreted immediately to urine, so, AUC24 might not rise and toxicities might be tolerable. In conclusion, clarithromycin inhibits MDR1 which is a membrane efflux transporter expressed in the small intestine and raise absorption of lenalidomide. Further studies are warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.