Abstract
The advent of next generation sequencing and emerging data demonstrating epigenetic dysregulation in cancer have greatly improved our understanding of common pathways underlying leukemogenesis in AML. Recent genomic studies have identified recurrent somatic mutations in cohesin complex genes in up to 13% of AML patients. The canonical role of the cohesin complex is to facilitate symmetrical DNA segregation of sister chromatids during mitosis, yet aneuploidy is an uncommon finding in AML. Thus the mechanisms by which cohesin mutations contribute to leukemogenesis in AML remain unknown. Here we investigate the role of the cohesin complex genes Scc1 and Smc3 in MLL-AF9 and AML1-ETO murine models of leukemia.
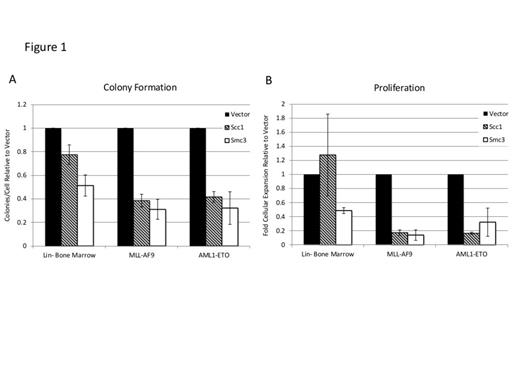
Most cohesin complex mutations in AML are predicted to be loss of function mutations leading to somatic haplo-insufficiency. To model haplo-insufficiency of cohesin genes we utilized lentiviral RNA interference targeted against Scc1 and Smc3 in wild type lineage-negative (Lin-) bone marrow or Lin- bone marrow transduced with MLL-AF9 or AML1-ETO. MLL-AF9 and AML1-ETO transduced Lin- bone marrow form tumor sphere-like colonies and demonstrate enhanced self-renewal and proliferation in vitro. Knockdown of Scc1 or Smc3 in Lin- bone marrow, MLL-AF9, and AML1-ETO transduced bone marrow was confirmed by real time quantitative PCR (RT-qPCR). Following knockdown of Scc1 or Smc3, MLL-AF9 transduced cells demonstrate diminished colony-forming capacity and fold cellular expansion in vitro compared to cells infected with empty vector (p-value <0.05, Figure 1). In AML1-ETO there was a trend towards fewer colony forming units following knockdown of Scc1 and Smc3 that was not statistically significant (p-value 0.36, 0.27, respectively, Figure 1A). However there was a more substantial block in the proliferative capacity of AML1-ETO cells following knockdown of Scc1 or Smc3 (p-value 0.006, 0.07, respectively, Figure 1B). Conversely, no change in colony formation or proliferation was seen in Lin- bone marrow following knockdown of Scc1 (Figure 1A), while knockdown of Smc3 was associated with a trend towards decreased colony formation (p-value 0.08) and decreased proliferation (p-value<0.01, Figure 1B). Due to cohesin’s canonical function in DNA segregation we investigated whether the observed findings were simply due to mitotic defects secondary to disruption of the cohesin complex. Propidium iodide staining was performed to assess cell-cycle phase in MLL-AF9 transduced cells and did not demonstrate an increase in aneuploidy following Scc1 or Smc3 knockdown.
Collectively, these data show that knockdown of cohesin subunits Scc1 or Smc3 have distinct differences between normal and malignant hematopoiesis. These differences depend upon the state of the cell transduced (Lin- bone marrow, MLL-AF9 or AML1-ETO) and which individual cohesin subunit is involved (Scc1 or Smc3). MLL-AF9 colony formation and proliferation were highly dependent upon intact Scc1 and Smc3. Whereas in AML1-ETO, colony formation was less affected by cohesin knockdown, but proliferation was impaired. In Lin- bone marrow, colony formation and proliferation were impaired by Smc3 knockdown, while self-renewal and proliferation were unchanged after knockdown of Scc1. The mechanism of impairment does not appear to be failure of mitosis resulting in aneuploidy and subsequent apoptosis. This suggests that the role of the cohesin complex in AML is dependent upon the underlying leukemogenic driver mutation, the specific cohesin subunit gene involved, and is unique from its role in normal hematopoiesis. Future studies will need to explore the specific pathways and interactions by which cohesin mutations contribute to malignant hematopoiesis.
Analysis of in vitro colony formation and proliferation after cohesin knockdown in Lin- bone marrow, MLL-AF9, and AML1-ETO.
Analysis of in vitro colony formation and proliferation after cohesin knockdown in Lin- bone marrow, MLL-AF9, and AML1-ETO.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.