Abstract
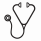
Introduction. A consensus-based approach to central nervous system (CNS) directed therapy has yet to emerge for pediatric patients with T-lineage acute lymphoblastic leukemia (T-cell ALL). We previously completed a meta-analysis of event-free survival (EFS) among studies using one of four prophylactic or therapeutic cranial radiation (CRT) strategies in pediatric T-cell ALL: (a) CRT for all patients (prophylactic strategy); (b) risk-directed CRT (studies that administered CRT to a subset of patients based on clinical characteristics such as age and white blood cell count at diagnosis (prophylactic strategy for a subset of patients); (c) CRT only for patients with involvement of the CNS with leukemia (CNS positive) at diagnosis only (therapeutic strategy); and (d) CRT omitted for all patients. We found that when adjusting for enrollment year, the EFS estimates were similar among studies that: a) administered CRT to all patients (prophylactic strategy); b) administered CRT to CNS positive patients only (therapeutic strategy); or c) omitted CRT for all patients. In contrast, the risk-directed approach was associated with inferior EFS. We hypothesized that an analysis of quality-adjusted life expectancy (QALE) might further clarify the role of CRT in current therapy for T-cell ALL. Informed by the results of our meta-analysis, we decided to compare the strategies of administering CRT to all patients (prophylactic strategy) as this has historically been the most common approach to CRT for T-cell ALL, to the approach of omitting CRT for all patients, a strategy that has been supported by observational studies. We compared the QALE for patients with T-cell ALL treated with or without CRT through the conduct of a decision analysis using a Markov model.
Methods. We performed a decision analysis using a Markov model to compare the strategies of administering CRT for all patients and omitting CRT for all patients, in terms of QALE, measured in quality-adjusted life years (QALYs). A Markov model is a state transition model in which a cohort of patients moves through different health states in the model. The entire cohort begins the model in the state, initial treatment, and then moves through the model (Figure 1). Each state is assigned a health utility score, which is a preference-based health-related quality of life weight, measured from 0-1 in which a score of 0 is equal to death and a score of 1 is equal to perfect health. We incorporated EFS estimates from a meta-analysis of observational studies for pediatric T-cell ALL published after 1999. Health utility scores from published estimates of acute lymphoblastic leukemia (ALL) subjects undergoing initial therapy, relapse therapy, and ALL survivors were assigned to the corresponding states in the Markov model. We included the contribution of increased deaths from late effects in our model, stratified by receipt of CRT, from standardized mortality ratios generated from mortality data from ALL survivors from the Childhood Cancer Survivor Study.
Results. We found that the expected value for those treated without CRT was 37.1 QALYs compared to 32.5 QALYs for those treated with CRT (4.6-year improvement in QALE). Treatment without CRT was the dominant strategy in sensitivity analyses. A Monte Carlo probabilistic sensitivity analysis that ran 100,000 simulated trials while simultaneously varying the plausible estimates for relapse rates, utilities, and standardized mortality ratios in survivors found that in 99.7% of the simulations treatment without CRT resulted in superior QALE. The Monte Carlo analysis also modeled the expected overall survival among subjects treated with either strategy (Figure 2) demonstrating the superior overall survival associated with a strategy of omitting CRT.
Discussion. Our analysis demonstrates that a strategy of omitting CRT for pediatric T-cell ALL leads to a QALE advantage of approximately 4.6 QALYs compared to a strategy that administers CRT for all T-cell ALL patients. This is a large benefit for a comparative treatment intervention compared to many published clinical decision analyses and cost-effectiveness analyses on diverse topics which typically show a life expectancy benefit of several months to several years for treatment interventions. This study lends further support to the movement to restrict or eliminate the routine administration of CRT for pediatric T-cell ALL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract