Abstract
Acute lymphoblastic leukemia (ALL) has historically been a challenging target for immunotherapy due in part to its tolerogenic effect on immune effector cells; however, CD19-targeted chimeric antigen receptor-modified T cells (CARTs) and bi-specific T cell engagers (BiTEs) show that immune tolerance to ALL can be circumvented. Unfortunately, both CD19-negative and positive relapses occur after treatment with either therapy, suggesting an approach targeting a single antigen may not be sufficient to eliminate ALL in all cases. Generating immune responses to multiple tumor antigens may be expected to provide superior relapse-free survival.
We previously reported an immune-mediated spontaneous remission model of B cell precursor ALL using leukemia cells derived from Eμ-ret transgenic mice. In this model, Eμ-ret leukemia cell lines expressing green fluorescent protein (GFP) and firefly luciferase (luc) in tandem initially engraft and expand in wild type (wt) mice but are subsequently eliminated by an immune response targeted to the neoantigens. This T cell-dependent remission provides long-term immune memory against ALL. In contrast, mice transgenic for GFP/luc were not able to reject the GFP/luc ALL, suggesting pre-existing immune tolerance plays a role in undermining immune responses to ALL.
In this study, we investigate the contribution of multiple antigens and the impact of antigen-specific tolerance on protection from leukemia outgrowth. Strikingly, the protective memory response against ALL is not dependent on the presence of neoantigens; mice given GFP/luc ALL cells who go into spontaneous remission do not develop disease when rechallenged with 106 wt ALL cells, while all naïve mice die of wt ALL by day 35 (p<0.0001). Furthermore, T cells from mice who have previously cleared GFP/luc ALL produce interferon gamma robustly when co-cultured with either GFP/luc ALL or wt ALL; whereas, naïve T cells do not. The neoantigen-induced immune recognition of wt ALL antigens appears to occur early, as all mice receiving wt ALL succumb to disease by day 35, while mice receiving GFP/luc ALL cells or a 1:1 mixture of GFP/luc and wt ALL fail to develop leukemia (p=0.0096).
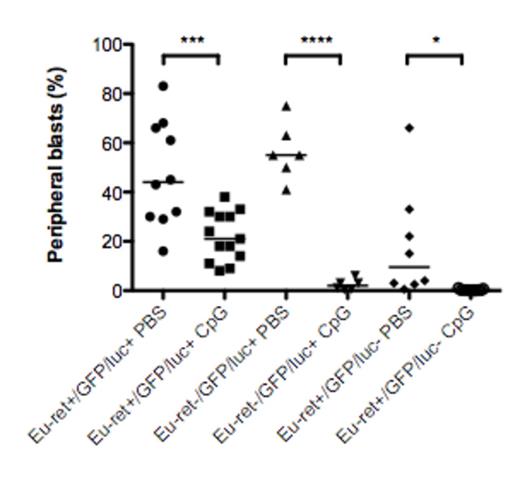
In contrast to wt mice, Eμ-ret transgenic mice show only delayed outgrowth of GFP/luc ALL compared to wt ALL (median survival 46 versus 26 days; p=0.0027) and are unable to reject the leukemia, leading to lethal disease burden in the majority of mice (p=0.014 versus wt mice with 100% survival at day 117). Given that Eμ-ret mice are likely tolerant to the transgene-related antigens, this result indicates that epitope spreading is essential to long-term protection. Finally, we evaluated the impact of non-antigen-specific immune stimulation on leukemia progression in a tolerized background. While the effect size decreased with increasing number of tolerizing antigens present in the mouse strain, treatment of GFP/luc ALL-bearing mice with the TLR9-agonist CpG oligodeoxynucleotides (CpG ODN) significantly reduced disease burden in all recipients, even GFP/luc/Eμ-ret transgenics tolerized to both transgene antigens and neoantigens (p=0.0013; Figure 1).
In the present study, we show that epitope spreading, a phenomenon with important implications for targeted immunotherapies, is essential for durable long-term protection from leukemia outgrowth. While generation of a robust multi-antigen anti-leukemia response may combat immune escape in the highly successful single antigen-targeted therapies, our results suggest that tolerance to leukemia-associated antigens will be a significant hurdle that may need additional therapeutic interventions to overcome. Our results show that non-antigen-specific immune stimulation with a TLR9-agonist achieves significant reduction in disease burden even in a fully immune tolerant model; importantly, the degree of tolerance in the transgenic models may be a better representation of the high immunologic bar for autologous ALL control in the clinical setting. This result provides further pre-clinical evidence that CpG ODN may be capable of generating clinically beneficial immune activity. Augmenting anti-leukemia activity by stimulating the innate immune system may protect against CD19 escape variant relapses, an important clinical problem in targeted immunotherapies.
Non-antigen-specific immune stimulation reduces leukemia burden in a tolerized background
Non-antigen-specific immune stimulation reduces leukemia burden in a tolerized background
Bassiri:GlaxoSmithKline: Equity Ownership. Barrett:Novartis: Research Funding. Grupp:Novartis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.