Abstract
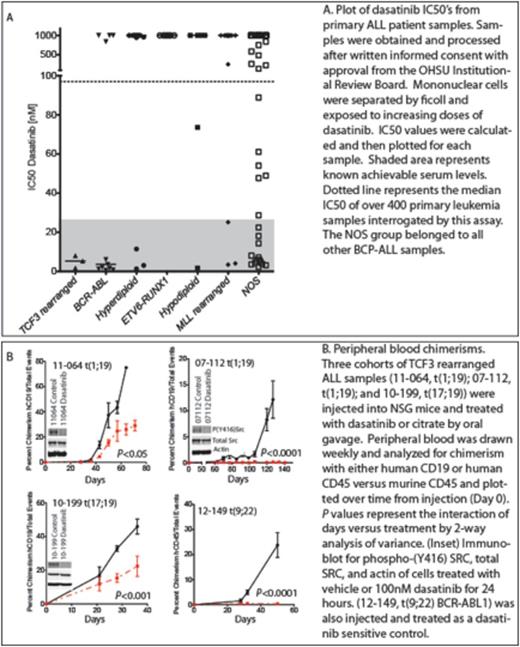
Transcription Factor 3 (TCF3) rearrangements are a recurring chromosomal abnormality in B-cell Precursor Acute Lymphoblastic Leukemia (BCP-ALL) occurring in approximately five percent of pediatric ALL. Historically, the majority of these patients carried a poor prognosis, but advances with more intensive cytotoxic chemotherapy have improved the survival rate while exposing patients to increased short and long-term toxicities. Two genetic rearrangements produce the chimeric transcription factors, TCF3-PBX1 t(1;19)(q23;p13) and a much rarer TCF3-HLF t(17;19)(q22;p13). Sadly, TCF3-HLF remains an extremely difficult disease to treat with few, if any known survivors. Although it is unknown how these translocations lead directly to disease, it is established that they do result in diseases arrested in a later stage of B-cell differentiation and pre-B cell receptor (pre-BCR) dependence. Recently, we highlighted the concept of targeting the pre-BCR pathway for therapeutic potential using dasatinib (Sprycel). Here, we further examine dasatinib effectiveness in the murine xenograft model for TCF3-rearranged ALL. Methods: Primary patient samples were obtained with written informed consent approved by the Institutional Review Board of Oregon Health and Science University and processed. Mononuclear cells were separated by Ficoll and exposed to increasing concentrations of dasatinib. Inhibitory Concentration of fifty-percent viability (IC50) was calculated for each sample. The median IC50 for over four hundred acute leukemic samples interrogated by this assay was calculated to approximately 100nM. Samples with IC50 values below 30nM were deemed hypersensitive to dasatinib. For xenografts, frozen viable primary patient samples were thawed and grafted via tail-vein into NOD/SCID/IL-2rgnull(NSG) mice 24 hours after sub-lethal irradiation with 200 cGy. Upon engraftment, and in vivo expansion, animals were euthanized and leukemic cells recovered from the spleen were then injected in secondary recipients. One week after injection the mice were divided into two groups and treated by oral gavage with dasatinib at 50mg/kg/dose daily or citrate control for 5 days per week. Treatment continued until the day of sacrifice (4-20 weeks). Peripheral blood engraftment was monitored weekly starting on week 3 by flow cytometry analysis using anti-human CD19 and CD45 (hCD19-APC, hCD45-FITC) versus anti-murine CD45 (mCD45-PerCP-Cy5.5). Flow cytometric data was analyzed using FACS/AriaIII. Results: Screening over one hundred BCP-ALL samples identified that approximately ten percent of these samples show hypersensitivity to dasatinib. TCF3-rearranged ALL and BCR-ABL1 ALL had a majority of samples with IC50's less than 10nM. Throughout all known subsets of ALL except ETV6-RUNX1, there also appeared to be individual samples that have IC50 values less than 30nM, suggesting significant sensitivity to this drug. Of these, three individual TCF3-rearranged ALL samples were identified and xenografted into NSG mice, expanded and injected into secondary recipients. All dasatinib treated cohorts showed significantly less leukemic peripheral blood chimerisms as compared to their vehicle control counterparts. Further, in vitro treatment of xenografted cells with dasatinib indicated inhibition of the pre-BCR by decrease in pan-phospho-SRC. Intriguingly, dasatinib did not completely abolish disease in all TCF3-rearranged ALL, suggesting other important mechanisms for cell viability. Conclusions: These studies show in vivo therapeutic benefits of dasatinib as treatment for TCF3-rearranged ALL, and open the possibility of adding this drug to their treatment. Further studies are underway to address the mechanisms of dasatinib sensitivity of other subsets of ALL identified in our screen in hopes of adding targeted therapies to their treatment.
Druker:Molecular MD: Consultancy, Equity Ownership, Scientific Founder. Some clinical trials on which I participate as PI or co-investigator utilize MolecularMD for molecular testing. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU. Other; Bristol-Myers Squibb: Clinical trial funding: PI and co-investigator on ARIAD clinical trials. OHSU has contracts with ARIAD to pay for patient costs, nurse and data manager salaries, and institutional overhead. I do not derive salary, or lab funds from these contracts. Clinical trial funding: PI and co-investigator on ARIAD clinical trials. OHSU has contracts with ARIAD to pay for patient costs, nurse and data manager salaries, and institutional overhead. I do not derive salary, or lab funds from these contracts. Other. Off Label Use: Dasatinib use as potential therapy in ALL.
Author notes
Asterisk with author names denotes non-ASH members.