Abstract
Precursor B cell (preB) acute lymphoblastic leukemia (ALL) is the most common cancer in children. Despite aggressive treatment, current approaches for preB ALL have significant limitations with a cure rate of only 30% in certain subtypes. In addition, long-term survivors are at risk for late effects that include development of secondary malignancies and toxicity to visceral organs. Therefore, there is a desperate need for more effective and less toxic therapy.
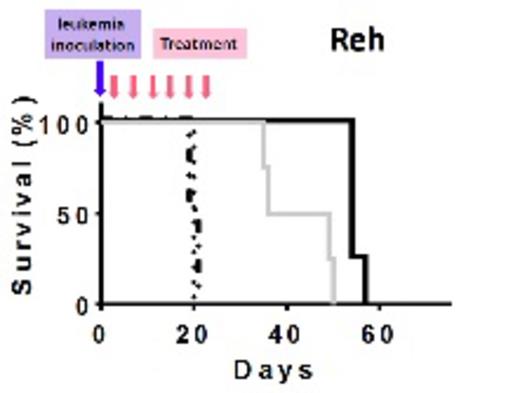
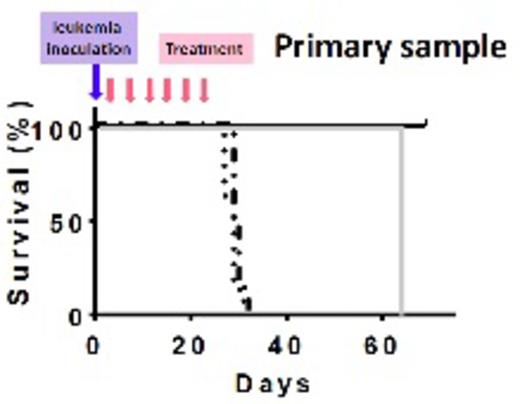
Treatments and outcome of the animals. Dash-dots: PBS, dots: MXD3 ASO and aCD22 Ab unconjugated, gray solid: aCD22 Ab-ASO conjugates at 0.2mg of Ab/kg/dose, and black solid: aCD22 Ab-ASO conjugates at 1mg of Ab/kg/dose
Treatments and outcome of the animals. Dash-dots: PBS, dots: MXD3 ASO and aCD22 Ab unconjugated, gray solid: aCD22 Ab-ASO conjugates at 0.2mg of Ab/kg/dose, and black solid: aCD22 Ab-ASO conjugates at 1mg of Ab/kg/dose
Previously, we demonstrated that the transcription factor MXD3 is a critical regulator of preB ALL cell proliferation. Knockdown of MXD3 expression in preB ALL cells resulted in cell death as determined by annexin V and caspase assays (Satake et al., British Journal of Haematology, in press). We hypothesize that targeted delivery of an MXD3 therapeutic to preB ALL cells will increase therapy effectiveness and decrease off target effects and toxicity, thus increasing the quality of life.
In the current study, we developed an antisense oligonucleotide (ASO) that specifically targets MXD3. Taking advantage of anti-CD22 antibody (aCD22 Ab), which specifically targets B cell ALL, we developed aCD22 Ab-MXD3 ASO conjugates as a potential combined phenotypic and molecularly targeted therapeutic. In in vitro tests, using the Reh ALL cell line, we confirmed successful delivery of the aCD22 Ab-MXD3 ASO compound to leukemia cells. This correlated with MXD3 knockdown at the protein level, and inhibition of leukemia cell growth. Cytotoxicity was tested on anonymized healthy donor normal blood cells, including CD34 positive hematopoietic stem cells (HSCs), B cells, and non-B cells from mobilized peripheral blood mononuclear cells. As expected, cytotoxicity was observed in normal B cells, but CD34 HSCs and non-B cells were unaffected. In combination with conventional preB ALL chemotherapy drugs (vincristine or doxorubin), we observed additive cytotoxic effects of aCD22 Ab-MXD3 ASO conjugates on cells in vitro.
We determined the compound effectiveness in pre-clinical xenograft animal models of preB ALL, using both the Reh cell line as well as a primary leukemia sample. Age matched female NOD/SCID/IL2Rg-/- (NSG) mice were randomly assigned to 4 treatment groups: 1) PBS, 2) MXD3 ASO and aCD22 Ab unconjugated at the equivalent dose of the high dose group, 3) aCD22 Ab-ASO conjugates at 0.2mg of Ab/kg/dose, and 4) at 1mg of Ab/kg/dose. Five million leukemia cells were inoculated either intravenously (iv) (Reh) or intra-bone marrow (primary cells) to each mouse. Twenty-four hours following leukemia inoculation, animals started receiving twice weekly iv treatments for 3 weeks. All mice treated with PBS and unconjugated MXD3 ASO & aCD22 Ab, died of leukemia at approximately day 21 in the Reh model and at day 30 in the primary leukemia model (Figure). Animals treated with aCD22 Ab-MXD3 ASO conjugates, either at low or high dose, had significantly prolonged survival both in the Reh (p<0.0084, n=4/group) and primary leukemia (p=0.0001, n=8/group) xenograft models (Log-rank (Mantel-Cox) test). Leukemia-related death was confirmed by necropsy. Harvested leukemia cells were HLA and CD22 positive. During treatment, the mice in all the treatment groups remained healthy and active, and did not lose weight. Toxicity was assessed in the primary leukemia model with weekly CBC and chemistry panels and revealed no significant toxicity.
In conclusion, we have demonstrated the therapeutic efficacy of the aCD22 Ab-MXD3 ASO conjugates in preB ALL. This is the first study to demonstrate effective direct ASO-conjugated monoclonal Ab-mediated delivery for the treatment of leukemia or other malignancies. Future studies will focus on the further characterization of the compound, effectiveness of combination therapy with standard chemotherapeutic drugs and toxicological profile.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.