Abstract

Background: Eastern Cooperative Oncology Group trial E1900 (E1900) showed that induction therapy with a high daily dose of daunorubicin (90 mg/m2) (DNR 90) improves survival in younger patients (pts) (<50 yrs) and intermediate (int) cytogenetic risk AML, but at 2 years of follow-up no benefit was seen in older pts (50-60 yrs), or in those with unfavorable cytogenetic risk or FLT3-ITD mutant AML (Fernandez et al. N Engl J Med 2009). Here we update results of E1900 with longer follow-up, focusing on the benefit of DNR 90 in cytogenetic and common molecular subgroups.
Methods: Overall survival (OS) was measured from randomization for induction therapy to death from any cause (censored at last contact). Hazard ratios (HR) for death were computed using univariate and multivariable Cox proportional hazards models; multivariable Cox models were adjusted for sex, age, hemoglobin level, leukocyte count, platelet count, and cytogenetic profile. All conclusions regarding the impact of DNR 90, unless noted, are similar based on univariate and multivariable analysis.
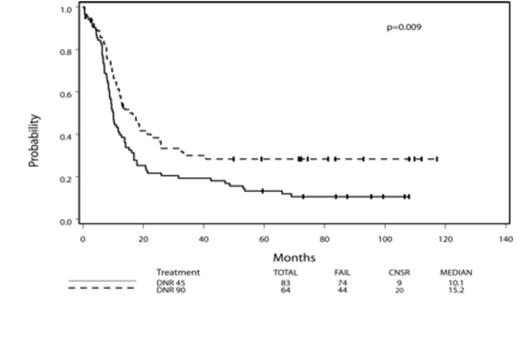
Results: Overall, 657 pts were enrolled with a median follow-up of 80.1 months. The HR for death in the DNR 90 group as compared with the standard-dose daunorubicin (45 mg/m2) (DNR 45) group was 0.74 (p=0.001). Pts <50 yrs benefited from DNR 90 (p = 0.002) while those >=50 yrs were not proven to benefit (p = 0.12). Pts with favorable (fav) and int. cytogenetic risk benefited from DNR 90 (p = 0.03 and p = 0.02, respectively). A benefit for pts with unfavorable cytogenetic risk was seen on multivariable analysis (p = 0.04).
Impact of DNR 90 by mutation status: The 3 most common mutations were FLT3-ITD (24%), NPM1 (26%), and DNMT3A (24%). AML pts with any of these 3 mutations benefited from DNR 90 (p = 0.009, p = 0.002, and p = 0.02, respectively). FLT3-ITD pts who received DNR 90 had a 4-yr OS of 31%. Benefit was seen in pts age 50-60 with FLT3-ITD or NPM1 mutation (p = 0.02 and p = 0.04, respectively). No benefit of DNR 90 was seen in a small cohort of pts with MLL-PTD (p = 0.06). Benefit of DNR 90 in FLT3-ITD, NPM1, and DNMT3A mutant AML was confirmed in the int. cytogenetic risk group.
Impact of DNR 90 on prognostic impact of NPM1: The presence of an NPM1 mutation conferred an improvement in OS in the DNR 90 group which was not seen in the DNR 45 group (p = 0.01 vs p = 0.3). This finding was confirmed in the int. cytogenetic risk group.
Conclusion:With median follow-up of over 6 years on E1900, we confirm that DNR 90 improves outcome in pts with fav/int cytogenetic risk, DNMT3A or NPM1 mutant AML, or age < 50 (Patel et al. N Engl J Med 2012). Additionally, we now demonstrate that DNR 90 additionally benefits pts with FLT3-ITD AML, and pts with unfavorable cytogenetic risk, regardless of age. Moreover, we show that the favorable prognostic impact of the NPM1 mutation is only present when pts receive DNR 90. Given the benefit of DNR 90 across all cytogenetic risk groups as well as common molecularly defined subgroups of AML, DNR 90 should be the standard for all pts up to age 60 who are candidates for induction chemotherapy.
HR for death by AML cohort.
| Subgroup . | N . | Univariate Model . | . | |
|---|---|---|---|---|
| DNR 45 . | DNR 90 . | HR (DNR 90/DNR 45) & 95% CI . | Wald P . | |
| All patients (n=657) | ||||
| Overall | 330 | 327 | 0.74 (0.61, 0.89) | 0.001 |
| Age < 50 yrs ³ 50 yrs | 188 142 | 172 155 | 0.66 (0.50, 0.85) 0.81 (0.62, 1.06) | 0.002 0.12 |
| Cytogenetic Favorable Intermediate Unfavorable | 38 232 59 | 51 212 63 | 0.51 (0.28, 0.93) 0.76 (0.61, 0.96) 0.79 (0.54, 1.16) | 0.03 0.02 0.22 |
| FLT3-ITD WT MUT | 215 83 | 241 64 | 0.74 (0.59, 0.92) 0.61 (0.42, 0.89) | 0.008 0.009 |
| MLL-PTD WT MUT | 290 16 | 296 15 | 0.70 (0.58, 0.86) 0.46 (0.21, 1.04) | 0.0004 0.06 |
| NPM1* WT MUT | 180 65 | 192 65 | 0.70 (0.55, 0.89) 0.50 (0.32, 0.78) | 0.003 0.002 |
| DNMT3A WT MUT | 177 61 | 194 58 | 0.66 (0.52, 0.85) 0.62 (0.41, 0.94) | 0.001 0.02 |
| Subgroup . | N . | Univariate Model . | . | |
|---|---|---|---|---|
| DNR 45 . | DNR 90 . | HR (DNR 90/DNR 45) & 95% CI . | Wald P . | |
| All patients (n=657) | ||||
| Overall | 330 | 327 | 0.74 (0.61, 0.89) | 0.001 |
| Age < 50 yrs ³ 50 yrs | 188 142 | 172 155 | 0.66 (0.50, 0.85) 0.81 (0.62, 1.06) | 0.002 0.12 |
| Cytogenetic Favorable Intermediate Unfavorable | 38 232 59 | 51 212 63 | 0.51 (0.28, 0.93) 0.76 (0.61, 0.96) 0.79 (0.54, 1.16) | 0.03 0.02 0.22 |
| FLT3-ITD WT MUT | 215 83 | 241 64 | 0.74 (0.59, 0.92) 0.61 (0.42, 0.89) | 0.008 0.009 |
| MLL-PTD WT MUT | 290 16 | 296 15 | 0.70 (0.58, 0.86) 0.46 (0.21, 1.04) | 0.0004 0.06 |
| NPM1* WT MUT | 180 65 | 192 65 | 0.70 (0.55, 0.89) 0.50 (0.32, 0.78) | 0.003 0.002 |
| DNMT3A WT MUT | 177 61 | 194 58 | 0.66 (0.52, 0.85) 0.62 (0.41, 0.94) | 0.001 0.02 |
Statistically significant test of interaction (p<0.2)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract