Abstract
Adult acute lymphoblastic leukemia (ALL) remains a therapeutic challenge with less than 40% long term survival. There is growing evidence that malignant diseases exert an immune editing effect which blocks antitumor immunity and permits tumor growth through immune evasion. Such tumor escape represents an obstacle for anticancer immunotherapy. In ALL such immune escape mechanisms are not well characterized. We therefore profiled cellular immunity in ALL, by characterizing the subsets of T cells, regulatory T cells (Treg), natural killers (NK) cells and γd T cells, using various functional markers including T cell exhaustion and NK cell activating or inhibitory molecules.
Forty ALL patients were included in the study. The median age was 39 y (range, 18-75). Thirty-six presented with B-lineage ALL and 4 with T-lineage ALL. Mononuclear cells were isolated from blood (n=19) or bone marrow (n=21) at the onset of leukemia or at relapse. The median infiltration of blasts was 85% (range 24-96%). Healthy donor peripheral blood (n=12) and bone marrow (n=9), from age and gender matched population, were simultaneously analyzed as controls. Extra-and intra cellular staining were performed using using antibodies directed against CD3, CD4, CD8, CD45, CD45, CD45RA, CD45RO, CCR7, CD95, CD27, CD19, CD14, CD127, CD25, Foxp3, Helios, αβTCR, HLA-DR, CD117, CD20, CD10, CD22, CD34, LAG3, PD1, PDL1, CD56, NKG2A, NKG2C, NKG2D, KIR2DL1, KIR2DL3, CD57, CD33, CD11b, CD15, CD38 and CD24. Data were acquired on a BD LSRFORTESSA flow cytometer.
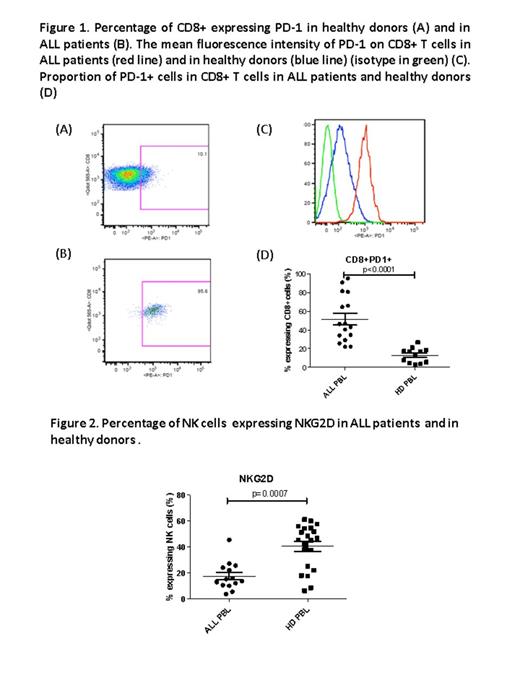
The expression of programmed cell death 1 (PD-1, CD279) receptor on CD8+T cells was significantly increased in blood and bone marrow of ALL patients compared to healthy donors (p<0.0001 and p=0.004, respectively) (Fig. 1). Focusing on the different subsets, CD8+ effector memory T cells significantly over-expressed PD-1 in blood and bone marrow of ALL patients compared to healthy donors (p=0.008 and p=0.04, respectively). Moreover, there was a significant positive correlation between PD-1 expression on CD8+ effector memory T cells and blast infiltration (R2=0.23, 95%CI 0.026-0.76, p=0.04). Expression of the co-inhibitory receptor lymphocyte-activation gene 3 (LAG-3, CD223) was similar in ALL patients compared to healthy donors. A significantly higher frequency of T regulators (CD25+, CD127 low, Foxp3+) was found in bone marrow microenvironment in ALL patients (4.3% versus 1.6%, p=0.02). Concerning γd T cells, frequency was similar in blood and bone marrow of ALL patients compared with healthy donors. There was a significantly lower frequency of CD56dimNKG2A+KIR-CD57- (p=0.02) in the bone marrow of ALL patients indicating a maturation arrest. Interestingly, expression of the activating receptor NKG2D which plays an important role in triggering the NK cell–mediated tumor cell lysis was significantly reduced in NK cells of ALL patients while no difference in NK cell expression of NKG2C was found(Fig. 2).
Adult patients with ALL show evidence of immune-editing of T cells and NK cells. This global immunosuppressive mechanism may contribute to the eventual escape of ALL from immune control. PD-1, overexpression, described in acute myeloid leukemia and chronic myeloid leukemia has been implicated in T-cell exhaustion and subsequent tumor immune evasion. Our data suggests similar immune escape mechanisms pertain in ALL. Effective antileukemia immunotherapy will require targeting one or more of these immunosuppressive pathways to achieve optimum results.
Fathi:Seattle Genetics, Inc.: Consultancy, Research Funding; Takeda pharmaceuticals International Co.: Research Funding; Exelixis: Research Funding; Ariad: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.