Abstract
Introduction: In ALL, minimal residual disease (MRD) is defined as the detection of leukemic cells in bone marrow by PCR or flow cytometry in the presence of hematological complete remission (CR). Patients with persistent/recurrent MRD after induction therapy have a higher risk of relapse than those with no detectable MRD. Effective treatment of patients with MRD aims to avoid hematologic relapse, reduce MRD load, and provide a bridge to subsequent HSCT.
Blinatumomab, an investigational BiTE® antibody construct, redirects CD3+ T cells to CD19+ target cells, resulting in serial lysis of CD19+ B cells. In a phase 2 study of blinatumomab in 21 patients with MRD+ ALL in first-line treatment, 80% of evaluable patients achieved a complete MRD response. BLAST, a confirmatory single-arm, phase 2 study evaluated efficacy, safety, and tolerability of blinatumomab in patients with MRD+ ALL in a larger population.
Methods: Adults (≥18 years) with B-precursor ALL in hematologic CR (<5% blasts in bone marrow) after ≥3 intensive chemotherapy treatments and with MRD ≥10-3 were eligible. Patients with current CNS pathology or extramedullary disease, previous allogeneic HSCT, or Ph+ ALL eligible for treatment with tyrosine kinase inhibitors were excluded. Blinatumomab 15 µg/m²/day was given by continuous IV infusion for 4 weeks, followed by a 2-week treatment-free period (1 cycle). Responders could receive ≤4 cycles of treatment or undergo HSCT after ≥1 cycle. Patients with hematologic relapse discontinued treatment. Rate of complete MRD response (absence of MRD confirmed with a sensitivity of at least 10-4 after 1 cycle) was the primary endpoint. MRD was assessed by allele-specific quantitative real-time PCR for clonal rearrangements of immunoglobulin or T-cell receptor genes by a central laboratory. Overall survival (OS), relapse-free survival (RFS), duration of complete MRD response, and incidence and severity of adverse events (AEs) were secondary endpoints. Per protocol, OS and RFS will be analyzed after 18 months minimum follow-up.
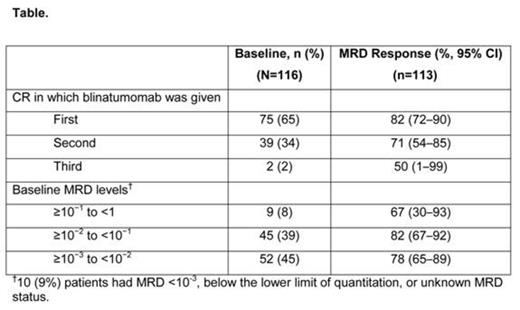
Results: 116 patients enrolled and received blinatumomab. Median (range) age was 45 (18–76) years; 15 (13%) patients were ≥65 years of age. 35% of the patients were treated in second or later remission (Table). As of February 2014, 106 patients had ended treatment: 74 had completed treatment (4 cycles or 1 cycle followed by HSCT) and 32 had discontinued treatment due to AEs, disease relapse, or investigators' decision; 79 patients were still alive and being followed. Three patients were excluded from the efficacy analysis: one had no central lab assay and two had assays with a sensitivity of 5×10-4. Eighty-eight patients (78% [95% CI, 69%–85%]) had a complete MRD response after 1 cycle of treatment. The lower CI bound exceeded 44% (the null hypothesis response rate), confirming that the study met its primary objective. Two additional patients had a complete MRD response after >1 cycle of blinatumomab; across all cycles the complete MRD response rate was 80%. The rate of complete MRD response did not differ significantly across baseline age, sex, line of treatment, and MRD burden categories (Table). All patients experienced ≥1 AE. AEs occurring in ≥20% of patients included pyrexia (88%), headache (38%), tremor (29%), chills (25%), fatigue (24%), nausea (22%) and vomiting (22%). Serious AEs (SAEs) occurred in 60% of patients; 59% and 27% of patients had grade ≥3 and grade ≥4 AEs, respectively. SAEs occurring in ≥5% of patients were pyrexia (15%), tremor (7%), aphasia (5%), encephalopathy (5%) and overdose (5%). Two fatal AEs occurred on treatment: subdural hemorrhage and atypical pneumonia (the latter was deemed treatment-related).
Conclusion: This is the largest prospective trial with an experimental compound in MRD+ ALL.Blinatumomab treatment resulted in complete MRD response across multiple patient demographics including patients in second-line treatment and those with high MRD burden. With a complete MRD response rate of 78%, the study met its primary objective. Among patients with a complete MRD response, 98% had a response within the first treatment cycle. In patients with MRD+ ALL following intensive therapy, rapid MRD response induced by blinatumomab has the potential to improve patient outcomes.
Goekbuget:Amgen Inc.: Consultancy, Honoraria, Research Funding. Off Label Use: This presentation will discuss the off-label use of blinatumomab, as this agent is not approved for use by the FDA, EMA or any other regulatory authorities.. Dombret:Amgen Inc.: Honoraria, Research Funding. Bonifacio:Amgen Inc.: Consultancy. Graux:Amgen Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees. Buss:Amgen Inc.: Reimbursement for participation in clinical studies, Reimbursement for participation in clinical studies Other; BMS: Travel support, Travel support Other; Novartis: Travel support Other; Pfizer: Reimbursements for participation in clinical studies, Reimbursements for participation in clinical studies Other. Bruggemann:Amgen Inc.: Consultancy, Research Funding. Stieglmaier:Amgen Inc.: Equity Ownership; Amgen Research (Munich) GmbH: Employment. Wessels:Amgen Inc.: Equity Ownership; Amgen Research (Munich) GmbH: Employment. Haddad:Amgen Ltd.: Employment. Zugmaier:Amgen Research (Munich) GmbH: Employment; Amgen Inc.: Equity Ownership. Nagorsen:Amgen Inc.: Employment, Equity Ownership, Related to blinatumomab Patents & Royalties. Bargou:Amgen Inc.: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract