Abstract
Background: Immune responses against Y-chromosomal encoded epitopes are well known as a driving force for both strong graft-versus-host (GvH) and graft-versus-leukemia (GvL) effects in female-into-male allogeneic hematopoietic stem cell transplantations (HSCT). Female donors with a history of pregnancies with male infants are likely to carry T lymphocyte based immune responses against minor histocompatibility antigens derived from the Y chromosome (H-Y antigens). A feto-maternal cell transfer and consecutive immunization of the mother forms the likely basis of this phenomenon. Interestingly, male individuals may carry T lymphocyte based immune responses against H-Y as well. So far, little is known about their frequency, their origin and their functionality particularly in light of allogeneic immunotherapy. Therefore, we screened a group of volunteer blood donors including men and women for H-Y responses. This group has been previously reported for immune responses against tumor-associated antigens (Lutz M et al, ASH 2012).
Material and Methods:After obtaining local ethical approval and written informed consent 114 HLA-A*02:01-positive healthy volunteer blood donors were enrolled in this scientific study including 38 nulligravidous women with a median age of 28 (21–53) years, 38 primi- or multiparous women with a median age of 46 (28–63) years and 38 men with a median age of 41 (21–56) years, respectively. Peripheral blood was drawn and peripheral blood mononuclear cells (PBMCs) were isolated using density gradient centrifugation. CD8+ T lymphocytes were isolated and stimulated with irradiated T2 cells (ATCC CRL-1992) which were pulsed with two different concentrations (0.1 and 10 µM) of the HLA-A*02:01-restricted minor antigen H-Y (FIDSYICQV) to distinguish between low- and high-avidity immune responses. After extraction of total RNA, Interferon gamma (IFNγ) mRNA expression was analyzed by quantitative reverse transcriptase polymerase chain reaction (RT-qPCR). IFNγ mRNA expression was normalized to CD8 mRNA levels and expressed as relative fold change compared to the irrelevant melanoma antigen Glycoprotein 100 (gp100). A two-fold change or more as compared to gp100 was considered as a positive result.
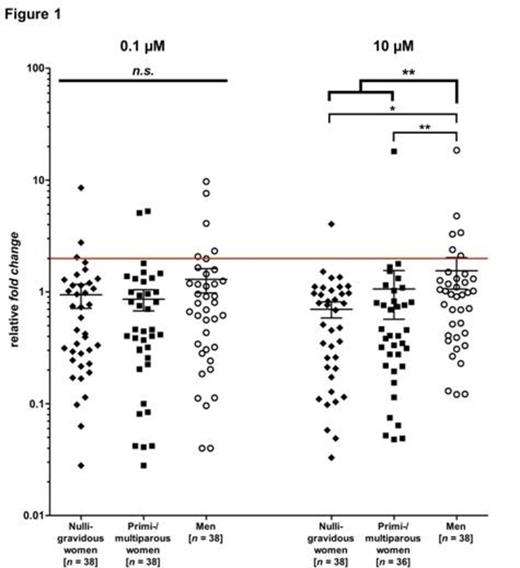
Results:Significant immune responses were detected in a small fraction of both women and men using two different peptide concentrations. Interestingly, women with a history of pregnancy did not show higher immune responses than those with no (known) history of pregnancy for both peptide concentrations. However, men showed the highest frequency of positive immune responses for both peptide concentrations. For the higher peptide concentration men showed significantly higher immune responses than nulligravidous (P < 0.05) or primi-/multiparous women (P < 0.01) or all women (P < 0.01). All results of this analysis are depicted in Figure 1 . The horizontal line represents the cut-off for positive immune responses (relative fold change of 2.0).
Conclusions: The results in women suggest that their immune responses – if not boosted by a further pregnancy or immunotransfer to an allogeneic individual – are short-lived. The detection of H-Y responses in nulligravidous women could be a result of unknown pregnancies in the past. We expected the highest frequency of significant immune responses against H-Y in primi- or multiparous women. However, to our surprise men showed the highest frequency and had significantly higher levels than women. This finding is in line with prior data where we found men to carry frequent immune responses against tumor-associated antigens such as WT1. At the end H-Y may serve as an auto-antigen to men suggesting that continuous gonadal expression of Y-chromosomal encoded antigens maintains these low-avidity autoimmune responses as previously described for cancer/testis antigens (Lutz M et al, ASH 2012). The transfer of these immune responses in a male-to-male HSCT may therefore contribute preferably to the GvL effect and thus be beneficial.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.