Abstract
The mutual interactions between the host immune system and cancer cells may either promote or control oncogenesis. Cancer immunotherapies remain viable approaches to sustain patient survival. However, positive clinical response of phase I/II clinical trials remains still to low. The Double negative T cells (DNTs) have emerged as new subset of T cells that contributes specifically to anti- tumor immunity since involved in immune regulation and tolerance acting as regulatory T cells (Treg) and/or cytotoxic T cells. DNTs express either αβ or γδ T-cell receptors (TCR) or lack CD4, CD8 and CD56. Functional studies showed that DNTs might have a direct in vitro anti-tumor activity against lymphoma and melanoma cells. However no data are available on their prognostic significance, modulation during the therapy response and their ability to kill tumor cells in cooperation with other immune cells such as DCs to explain their role in human anti-lymphoma immunity. Knowledge of the DNTs role in tumor surveillance and as prognostic factor for clinical outcome has a strong clinical valence and might allow us to design new approaches of therapeutic strategy.
The aim of this study was to evaluate the DNTs during therapy response in lymph nodes (LN), bone marrow (BM) and peripheral blood (PB) of both lymphoma (Ly), Renal Cell Carcinoma (RCC) and Metastatic Melanoma (MM) patients (pts) in order to assess their role on clinical outcome, progression and tumor surveillance.
PB and BM samples of 90 Ly pts, with NHL and cHL were collected at diagnosis and during the follow-up (1-6-12 months after chemo- or immuno-chemotherapy therapy). PB samples of 16 healthy donors were collected as control. Twenty fresh LN tissues from pts clinically suggestive of Ly were quickly processed. To evaluate the interaction of DNTs with tumor burden either 22 samples from RCC pts and 30 samples from MM pts were collected. The ontogeny, functional attitude and TCR clonality of DNT subsets (TCRαβ+ and TCRγδ+) were evaluated by following antibodies: CD3, CD4, CD8, CD56, CD45, TCRαβ, CD45Ra, CD45Ro, CCR7, CD27, CD28, CD30, CD69, GITR, CD95, CD178, CD152, IFN-γ, TNF-α, perforin and granzyme B. For functional studies, DNTs were purified from PBMCs through a negative selection and then cultured for 2 weeks. Data were acquired by 8-colour flow cytometer and analysed using Kaluza software. Other immune cells such as DCs, MDSCs and Treg was also detected to evaluate the correlation with DNTs. 7 cases of LNs received a finally diagnosis of reactive follicular hyperplasia (RFH) so they were considered as controls. All pts provided their informed consent in accordance with the Declaration of Helsinki.
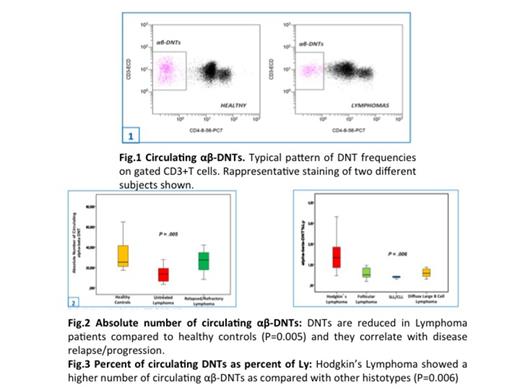
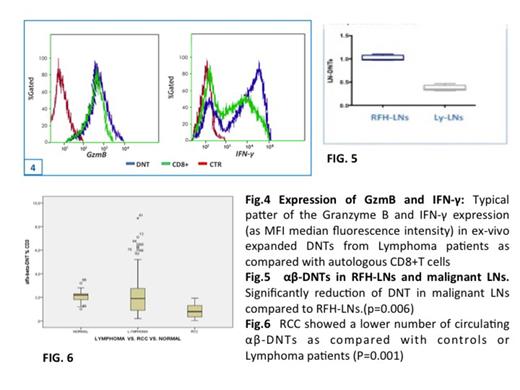
We observed a significant decrease (p =0.006) of circulating αβ-DNTs in untreated Ly pts (20.5 cells/ul ± 4.8) as compared with healthy controls (31.3 cells/ul ± 3.4) (fig.1-2) and their number seemed to be modulated during the follow-up. Their frequency in 1-month post-cht or disease relapsed pts was significantly decreased (p=0.006) as compared with the diagnosis as well as when compared indolent with aggressive histotypes (p=0.0001). In HL pts the frequency of αβ-DNT was significantly increased as compared with other histotypes (p=0.005) (Fig.3) Furthermore, the αβ-DNTs in LNs of Ly pts were significantly reduced as compared to to RFH-LNs (p=0.006) (fig.5) and are related to the aggressiveness of the disease. When evaluated either in MM and RRC pts the αβ-DNTs were significantly decreased (p=0.001) as compared with healthy controls and more interestingly when compared with Ly pts (p=0.001) given the greater immunological impairment of RCC/MM tumor burden (fig.6). Finally, ex vivo expanded DNTs acquired an immunomodulatory cytokine profile, characterized by the secretion of IFN-gamma and granzyme B, which are known as central components of anti-tumor immune responses supporting the hypothesis of αβ-DNT potential use in immunotherapeutic strategies (fig.4)
Our preliminary data, showed an inverse correlation between the frequency of circulating αβ-DNTs and the tumor condition as well as that they could play an important role in both the development and the progression of lymphomas. This is the first study that compares the frequency of αβ-DNTs in three different pathologies correlating to tumor burden. In addition, it is likely that ex-vivo expanded DNTs exert an anti-tumor activity thus suggesting their possible use as a new strategy for adoptive immune-therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.