Abstract
Background and objective: Autoimmune hemolytic anemia (AIHA) is mediated by warm and cold-reactive autoantibodies, with the treatment approach depending on the type of antibody. Corticosteroids, immunosuppressive agents and splenectomy are the commonly used therapies for AIHA. Over the last decade, rituximab has emerged as a preferred second line agent for treatment of AIHA, though evidence supporting its use in AIHA, especially cold antibody-mediated AIHA, is limited to case reports and small case series. We therefore conducted a 13 year retrospective cohort study of patients treated for AIHA at the Cleveland Clinic to evaluate the outcomes of patients with this disorder treated with rituximab.
Methods: Adult patients presenting with newly-diagnosed diagnosis or relapsed AIHA were included.The clinical presentations, type of AIHA (warm, cold, mixed), treatment and outcomes were recorded. Frequencies of complete responses (CR) (non-transfused hemoglobin of ≥ 12 mg/dL, hemoglobin increase ≥ 2 mg/dL, and normalization of hemolytic markers), and partial responses (PR) (non-transfused hemoglobin of ≥ 10 mg/dL with hemoglobin increase of ≥ 2 mg/dL) were recorded. Time to relapse was estimated using the Kaplan Meier method. A p value of ≤ 0.05 was considered significant for all analyses.
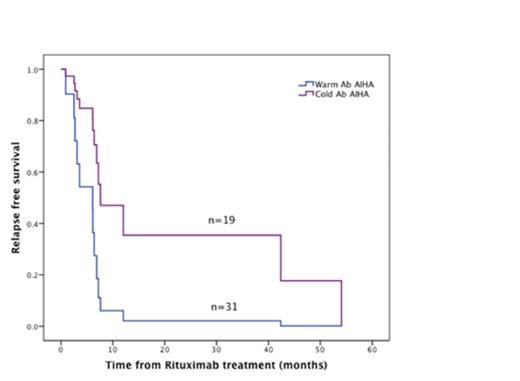
Results: We identified 180 patients with AIHA of which 50 patients (31 with warm AIHA and 19 with Cold AIHA) were treated with rituximab. The rates of primary and secondary AIHA (associated with autoimmune disease, malignancy or drugs) were similar in patients with warm and cold AIHA (p=0.660). Rituximab was used as first line therapy in combination with steroids in 15 patients and as second/third line treatment in 35 patients. Only 17 patients received rituximab monotherapy while 33 received combination therapy with steroids. Overall response rates for Rituximab were 74% (17% CR, 57% PR) and 80% (27% CR, 53% PR) for patients with warm and cold AIHA respectively (p =0.877). Median time to response was 0.7 months in warm AIHA and 1.1 months in cold AIHA (p=0.112). Seven (22.5%) patients with warm AIHA but none with cold AIHA required maintenance steroids after Rituximab treatment. Relapses occurred in 9 (29%) patients with warm and 5 (26%) patients with cold antibody-mediated AIHA after rituximab treatment (p=0.154). The median time to relapse was 8.5 months for warm and 55.2 months for cold AIHA (p=0.044) (Figure 1).
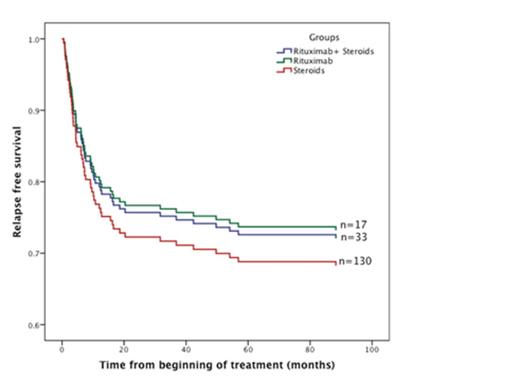
Patients who received rituximab with or without concomitant steroids demonstrated lower rates of relapse (26%) than those who received corticosteroids alone (32.3%) [Relative Risk 1.35 (1.09-2.82), p=0.047] (Figure 2). There were no major adverse events due to Rituximab over a median follow up of 54 months.
Conclusion: Rituximab is highly effective in both warm and cold AIHA with higher response rates and lower relapse rates than corticosteroid therapy alone. In this cohort, there was a high incidence of durable responses, especially in cold antibody-mediated AIHA.
Kaplan Meier curves showing relapse-free survival after rituximab for patients with warm and cold antibody AIHA. The median time to relapse was 8.5 months for warm and 55.2 months for cold AIHA (p=0.044).
Kaplan Meier curves showing relapse-free survival after rituximab for patients with warm and cold antibody AIHA. The median time to relapse was 8.5 months for warm and 55.2 months for cold AIHA (p=0.044).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.