Abstract
Introduction
Acute chest syndrome (ACS) is a severe complication of sickle cell disease (SCD) and a leading cause of hospitalisation and mortality. ACS is defined as a new pulmonary infiltrate on chest radiograph with associated fever or respiratory symptoms. The highest incidence of ACS is in children; occurring in up to 50% in the first decade and peaking in incidence between 2 and 5 years of age.
The mainstay of treatment of acute ACS is supportive and clinical management of ACS varies between centres in the type of blood transfusion (top-up versus exchange), post transfusion percentage sickle cells (HbS%); type of ventilatory support and guidelines regarding escalation of care. Additionally, there are controversies in the use of bronchodilators and corticosteroids in the acute management of ACS. To allow trials to be undertaken, it is important to first assess the current practice of the acute management of ACS in children across paediatric centres in the UK and Europe.
Methods
An electronic survey was devised based on the currently available literature on management of children with ACS. The survey was sent to major centres in the UK, France, Ireland, Netherlands and Belgium who are involved in clinical management of Paediatric SCD. A retrospective single-centre audit of the management of Paediatric ACS was also undertaken in the researchers’ institution over a 24-month period.
Results
1. Multinational survey
The survey was sent to 48 centres of which 20 centres responded (38%). 68% (n=13), of centres treat less than 10 cases of ACS per year.
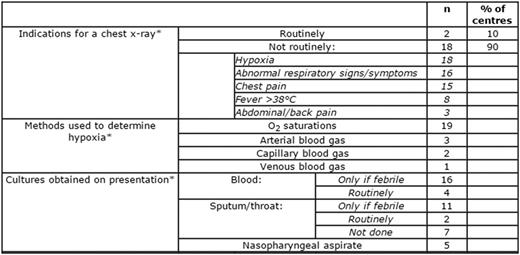
There was variation in diagnostics (table 1), use of incentive spirometry, fluids and antimicrobials (table 2), indications for transfusion (table 3) and in the escalation of care (table 4) between centres.
– Intervention
| . | n . | % of centres . | |
|---|---|---|---|
| Incentive spirometry given | Yes | 15 | 75 |
| No | 5 | 25 | |
| Fluid given | 80% | 1 | 5 |
| 100% | 14 | 70 | |
| 150% | 4 | 20 | |
| Antibiotics routinely prescribed to treat ACS* | Clarithromycin | 16 | |
| Cefuroxime | 11 | ||
| Ceftriaxone | 6 | ||
| Co-amoxiclav | 3 | ||
| Are antivirals prescribed? | Yes | 6 | 31 |
| No | 13 | 68 | |
| When are bronchodilators given* | To wheezy patients | 10 | |
| To all patients with ACS | 6 | ||
| Asthmatics | 4 | ||
| Not routinely given | 3 | ||
| Are corticosteroids routinely given | Yes | 1 | 5 |
| No | 17 | 85 | |
| Only to known asthmatics | 2 | 10 | |
| . | n . | % of centres . | |
|---|---|---|---|
| Incentive spirometry given | Yes | 15 | 75 |
| No | 5 | 25 | |
| Fluid given | 80% | 1 | 5 |
| 100% | 14 | 70 | |
| 150% | 4 | 20 | |
| Antibiotics routinely prescribed to treat ACS* | Clarithromycin | 16 | |
| Cefuroxime | 11 | ||
| Ceftriaxone | 6 | ||
| Co-amoxiclav | 3 | ||
| Are antivirals prescribed? | Yes | 6 | 31 |
| No | 13 | 68 | |
| When are bronchodilators given* | To wheezy patients | 10 | |
| To all patients with ACS | 6 | ||
| Asthmatics | 4 | ||
| Not routinely given | 3 | ||
| Are corticosteroids routinely given | Yes | 1 | 5 |
| No | 17 | 85 | |
| Only to known asthmatics | 2 | 10 | |
– Transfusion
| . | n . | % . | |
|---|---|---|---|
| Indication for transfusion in patients with ACS* | Worsening anaemia with respiratory symptoms | 17 | |
| Worsening respiratory symptoms | 12 | ||
| O2 saturations 3% below usual baseline | 7 | ||
| Target post transfusion HbS% | <20% | 2 | 10 |
| <30% | 14 | 73 | |
| <50% | 3 | 15 | |
| . | n . | % . | |
|---|---|---|---|
| Indication for transfusion in patients with ACS* | Worsening anaemia with respiratory symptoms | 17 | |
| Worsening respiratory symptoms | 12 | ||
| O2 saturations 3% below usual baseline | 7 | ||
| Target post transfusion HbS% | <20% | 2 | 10 |
| <30% | 14 | 73 | |
| <50% | 3 | 15 | |
– Escalation of care
| . | n . | % of centres . | |
|---|---|---|---|
| Indication for non-invasive ventilation (NIV)* | Worsening hypoxia | 15 | 75 |
| Severe dyspnoea | 13 | 65 | |
| Increasing hypercapnia causing respiratory acidosis | 11 | 55 | |
| Centre does not offer NIV | 4 | 20 | |
| Mode of respiratory support offered* | High flow oxygen | 14 | 70 |
| Continuous positive airway pressure (CPAP) | 10 | 50 | |
| Optiflow TM | 7 | 35 | |
| Other (CNEP, BiPAP) | 3 | 15 | |
| Unknown | 1 | 5 | |
| . | n . | % of centres . | |
|---|---|---|---|
| Indication for non-invasive ventilation (NIV)* | Worsening hypoxia | 15 | 75 |
| Severe dyspnoea | 13 | 65 | |
| Increasing hypercapnia causing respiratory acidosis | 11 | 55 | |
| Centre does not offer NIV | 4 | 20 | |
| Mode of respiratory support offered* | High flow oxygen | 14 | 70 |
| Continuous positive airway pressure (CPAP) | 10 | 50 | |
| Optiflow TM | 7 | 35 | |
| Other (CNEP, BiPAP) | 3 | 15 | |
| Unknown | 1 | 5 | |
2. Single-centre retrospective audit
Forty-six patients were admitted to St Mary’s Hospital with ACS during the period of the study. The median age was 10 years.
Ninety per cent (n=36) of patients had a chest x-ray on admission. The average Hb on admission was 76 g/L. Eighty-nine per cent (n=33) of patients had blood cultures taken on admission; of which two were positive (Acinetobacter and Micrococcus spp). Non-invasive ventilation was given to 47% (n=18) of patients. Forty-two per cent (n=24) of patients were given at least one blood transfusion with average post-transfusion HbS% of 28.5.
Conclusion
This study reflects a wide variation in management of ACS in centres across the UK and Europe, with a focused audit of one centre in London. Consensus was reached in provision of incentive spirometry and antibiotic use, but practice varied widely in indications for blood transfusion, anti-viral agents and bronchodilators.
A multi-centre prospective trial is therefore required in ACS management to establish optimum management based on patient outcome.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.