Abstract
Background: Invariant NKT (iNKT) cells are a lymphocyte subset that can be rapidly activated to produce cytokines and fuel tissue inflammatory responses that are implicated in sickle cell disease (SCD) pathogenesis. In mouse models of SCD, interrupting the activation of iNKT cells decreases tissue injury. Patients with stable SCD, defined as no recent vaso-occlusive crisis (VOC), illness or transfusion, were enrolled into a phase 1 study utilizing an anti-iNKT cell monoclonal antibody, NKTT120, aimed at establishing a safe and effective dose to reduce or deplete their blood and tissue iNKT cells. Since monitoring iNKT cell response to our depleting antibody is a critical measure of both efficacy (iNKT cell depletion) and recovery (iNKT cell return to peripheral circulation) we assessed stability of the fraction of activated iNKT cells as well as their percentage as a fraction of total CD3+ lymphocytes measured over two time points.
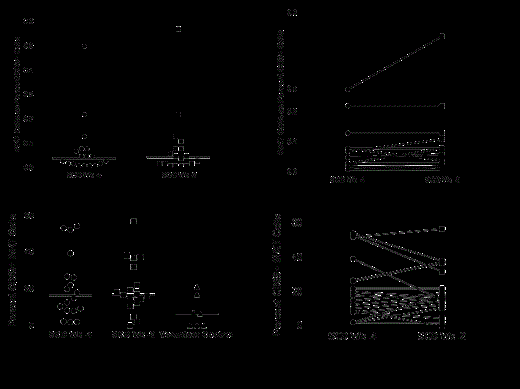
Methods: In 18 adults (12 males/6 females, 21-43 years) with stable SCD, twomeasures of blood iNKT cells were obtained at a 2 week interval during screening for entry into a Phase Ib clinical trial. In addition, we also evaluated a single sample from 7 male adult volunteers (18-31 years). Lymphocyte profiles were analyzed by FACS. A minimum of 100,000 CD3+ events were acquired to ensure a robust measure of iNKT cells (identified by TCR Va24/Vb11expression). The lower limit of quantitation of the assay was 0.01%. The iNKT cells are expressed as a percentage of CD3+ T cells. The iNKT cell activation status was defined as the percent positive for the activation marker CD69.
Results: The median iNKT cell percent of CD3+ T cells was 0.04% (range 0.0-0.5) when sampled 4 weeks before enrollment and was 0.04% (0.0-0.57) when sampled 2 weeks before enrollment (A). The median percent activated iNKT cells in the Phase 1 population was 17% (range 2-54%) at week -4 and 17% (range 0-57%) at week -2. Normal volunteers had a mean of 7% (range 0-21%) (C). The iNKT measurements done at a 2 week interval within a patient at steady state were highly correlated (r=0.91, p<0.001) (B, D). Fifteen of the SCD adults both at week -4 and week -2 had active iNKT cells that were at or exceeded the mean for the normal volunteer group. However, only two normal adults were at or near the SCD group mean with the highest normal at 21% vs. 57% for the SCD group (C).
Conclusions: In adult patients with stable SCD, the iNKT cell fraction of the total CD3+ population that are CD69+ is elevated compared with normal volunteers. This finding supports the hypothesis that iNKT cells, via their ability to release inflammatory mediators and activate other immune cells, are implicated as one mediator of the chronic inflammation associated with SCD disease. In addition, this population of activated iNKT cells is stable on repeat measure, suggesting that a single measurement is representative of the iNKT concentration and activation state over a one month time period. These data have important clinical implications since iNKT cell measurement is an important component in our clinical studies of iNKT cell depletion and recovery.
Mazanet:NKT Therapeutics: Employment. Schaub:NKT Therapeutics Inc: Employment, Equity Ownership. Scheuplein:NKT Therapeutics Inc: Employment, Equity Ownership. Eaton:NKT Therapeutics: Employment. Mashal:NKT Therapeutics Inc: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.