Abstract
MicroRNA (miRNA) regulation of dendritic cell (DC) development and function is not fully understood. We have previously reported 27 differentially expressed miRNAs during human monocyte differentiation into immature DCs (imDCs) and mature DCs (mDCs). Here, we aimed at uncovering the functional role of miR-146a and miR-146b (miR-146a/b) during this differentiation process.
To investigate miR-146a and miR-146b expression during human monocyte differentiation into imDCs and mDCs, monocytes were differentiated into imDCs with GM-CSF and IL-4 and matured with IL-1β, IL-6, TNF-α, and PGE2. We found by qRT-PCR that expression of miR-146a/b was dramatically increased upon monocyte differentiation into imDCs (miR-146a, 10-fold; miR-146b, 37-fold at day6; n=4; p value of Student t test<0.05) and mDCs (miR-146a, 51-fold; miR-146b, 79-fold; n=4; p<0.005). Up-regulation of miR-146a/b in mDCs was predominantly mediated by IL-1β but not IL-6, TNF-a, or PGE2 (n=3; p<0.01).
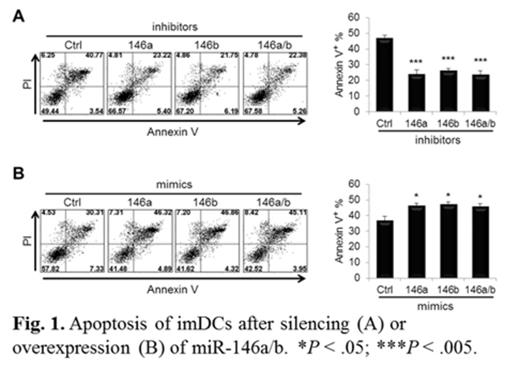
DC apoptosis is important for self-tolerance and immunity. We then evaluated the effect of altering miR-146a/b expression levels on DC apoptosis by Annexin V/PI staining. Silencing of miR-146a, miR-146b or both in imDCs (Fig. 1A) and mDCs significantly prevented DC from apoptosis (Fig. 1A; miR-146a, 24%±3.1; miR-146b, 26%±1.7; miR-146a/b; 23%±2.8 vs control, 46%±2.3 of Annexin V+ populations; n=8; p<0.005) whereas overexpression of miR-146a, miR-146b or both in imDCs (Fig. 1B) and mDCs significantly increased the proportion of apoptotic cells (Fig. 1B; 46%±1.9, 47%±2.1, 45%±2.3 vs 36%±3.3; n=6; p<0.05). These results indicate that miR-146a/b may function as pro-apoptotic regulators during human monocyte differentiation into imDCs and mDCs.
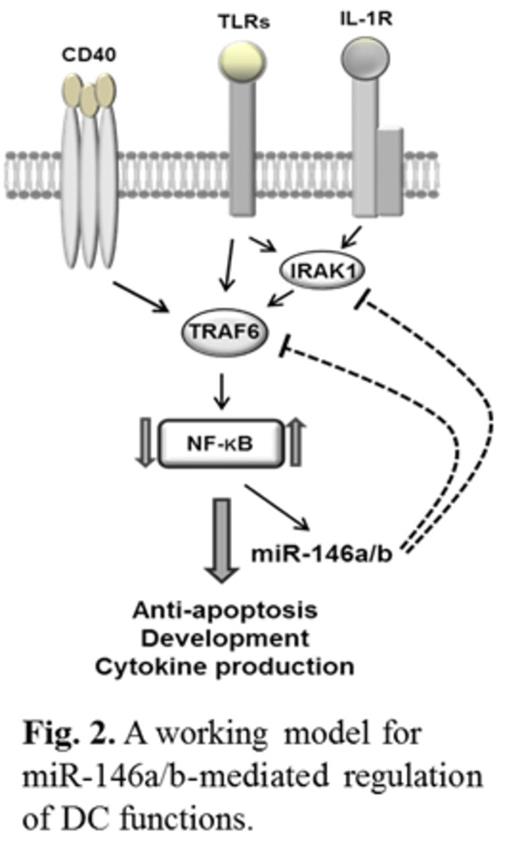
It is known that the NF-κB pathway regulates DC development, function and survival, and that TRAF6 and IRAK1 are major signal transducers in the NF-κB pathway. In addition, both TRAF6 and IRAK1 are known target genes of miR-146a. Indeed, miR-146a/b expression in imDCs and mDCs was inversely correlated with TRAF6 and IRAK1 mRNA and protein expression (n=4; p<0.05). Furthermore, siRNA silencing of TRAF6 and/or IRAK1 in imDCs and mDCs significantly enhanced DC apoptosis (n=4; p<0.05). By contrast, lentivirus overexpression of TRAF6 and/or IRAK1 promoted DC survival compared to control lentivirus transduced cells (n=2; p<0.05). To confirm that miR-146a/b-induced human DC apoptosis is involved in suppression of the NF-κB pathway, at least in part through down regulation of the NF-κB signaling transducers TRAF6 and IRAK1, we examined the protein level of IκB as a negative regulator of NF-κB and Bcl-2 as a known downstream anti-apoptotic molecule of the NF-κB pathway. Silencing of miR-146a/b in imDCs and mDCs significantly decreased IκBα and increased Bcl-2 expression whereas overexpression of miR-146a and/or miR-146b or silencing of TRAF6 and/or IRAK1 significantly increased IκBα and decreased Bcl-2 expression in imDCs and mDCs (n=4; p<0.01). These results indicate that miR-146a/b modulate DC apoptosis through inhibition of NF-κB activation via targeting TRAF6 and IRAK1.
Next, we investigated whether miR-146a/b regulates pro-inflammatory cytokine production in DCs. We found that IL-12p70, IL-6 and TNF-α production were significantly enhanced after miR-146 and/or miR-146b silencing during DC maturation (n≥2; p<0.05), although altering miR-146a/b expression had little effect on DC maturation (n=6). By contrast, IL-12p70, IL-6 and TNF-α production was highly reduced after miR-146a and/or miR-146b overexpression (n≥2; p<0.05).
In conclusion, we have demonstrated three important findings in this report. First, expression of both miR-146a and miR-146b is up-regulated during human monocyte differentiation into imDCs and mDCs. Secondly, although miR-146a and miR-146b do not appear to play a role in DC maturation, they may be critical regulators of DC apoptosis and cytokine production. Thirdly, mechanistically, miR-146a/b targets TRAF6 and IRAK1, leading to inhibition of NF-κB and reduced expression of Bcl-2. We thus demonstrate for the first time that miR-146a/b regulates human DC apoptosis and cytokine production, uncovering a new negative feedback mechanism for miR-146 in controlling overstimulation of the immune responses (Fig. 2).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.