Abstract
Background: Thrombocytopenia in Immune Thrombocytopenia (ITP) results from a combination of increased platelet destruction and reduced production, both often secondary to anti-platelet antibodies. Absolute immature platelet fraction (A-IPF) is a measure of young reticulated platelets in peripheral blood, and therefore provides an assessment of platelet production. Decreased platelet production in patients with ITP has been addressed by the recently developed Thrombopoietin Receptor Agonists (TPO-RA), which stimulate megakaryopoiesis and thereby, in responders, increase platelet production to a level which overcomes platelet destruction. The majority of ITP patients will respond to these agents, but which ones will respond is not known.
Aims: To explore
A) whether baseline A-IPF levels are associated with platelet response to TPO–RA, and
B) whether antibodies to platelet glycoproteins 1b or β3 influence the platelet response to TPO-RA.
Methods: Platelet counts and A-IPF values were collected from patients treated with TPO-RA (n=91) at Weill Medical College of Cornell University until 2013. All available counts were included, and the median count for each month was determined pre-treatment, and at 1, 2, 3, 4, 5, and 6 months. Patients were divided by whether (n=20) or not (n=71) they often received rescue therapy (i.e. IVIG and/or daily steroids >10mg).
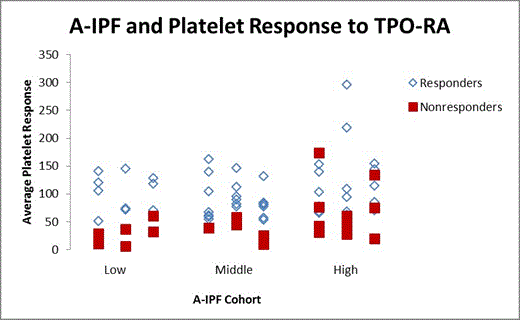
The 71 patients who received minimal or no rescue treatment were deemed responders if the average of their six median monthly platelet counts doubled from baseline and was >50x109/L. Based on pretreatment A-IPF, patients were stratified into three cohorts: low (0-25), middle (26-40), and high (41+). Clinical variables such as age, splenectomy status, duration of ITP, and gender were also studied. The 20 “rescue” patients were similarly analyzed.
The 84 patients with available sera had their antibody levels measured to GP1b and β3 by Elisa by Dr. Ni. Samples were sent to the lab and analyzed without knowledge of response status or associated A-IPF level. Patients were divided into four categories for analysis of their antiplatelet antibodies: positive for anti-ß3 antibody, positive for anti-GP1b, positive for both, or negative for both.
Fisher’s exact, student t-, and chi-square tests were used to analyze differences in response to TPO-RA among patient groups, A-IPF levels, and anti-platelet antibodies.
Results:
71 Patients with Minimal Rescue Therapy
Fifty-nine of the 71 patients responded to TPO-RA. There was no significant difference in A-IPF values for responders and non-responders (p=0.95; Figure 1). A significant positive correlation was observed between increase in A-IPF over the 6-month period and response rate to TPO-RA (p=0.009). Age, gender, duration of ITP, and splenectomy status neither correlated with response rate, nor trended with A-IPF cohorts.
20 Patients with Rescue Therapy
For the 20 patients who often received rescue therapy, low response rates were seen in the low A-IPF cohort and high response rates in the higher A-IPF cohorts. The percent of responders to treatment was higher in patients with chronic ITP than with newly diagnosed and persistent ITP (p= 0.04). Age, gender, and splenectomy status showed no relationship with response to TPO-RA. In comparison with the 71 patient group, the 20 patients presented lower response rates in all groups, with the exception of females.
Anti-platelet Antibodies
There was a weak correlation between A-IPF and anti-GP1b antibody levels (R=0.225), demonstrating that antibodies to GP1b may have a specific impact on platelet production.
Patients who did not have detectable antibody to either GP1b or β3 responded better to TPO-RA than patients who tested positive for both Anti-GP1b and Anti-ß3 (p=0.003). The strongest correlation was observed between level of anti-GP1b and response to TPO-RA (p<0.001; Figure 2).
Conclusion:
Rather than baseline A-IPF levels or clinical variables, the best predictor of good response to TPO-RA therapy was the absence of anti-GP1b and anti- β3 platelet antibodies, especially anti-GP1b. An explanation for the dominant effect of antibodies to GP1b is that stimulating megakaryopoiesis with TPO-RA would not effect a platelet increase as these antibodies may block proplatelet extension, preventing platelet release into the circulation. This may also explain the effect of anti-GP1b on response to steroids and IVIG.
Off Label Use: Eltrombopag is a thrombopoietin receptor agonist approved for the treatment of thrombocytopenia in adults with chronic ITP. Use in children and adolescents will be discussed.. Bussel:Amgen: Equity Ownership; GSK: Equity Ownership; Amgen: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; GSK: Research Funding; Sysmex: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.