Abstract
Introduction The Intercept Blood System (IBS) is currently approved in 40 countries to prevent transfusion-transmitted diseases. It is mandatory in Switzerland since 2012. Our aim was to study the effects of this treatment on platelet function and survival and to investigate the underlying mechanisms.
Methods 10 platelet apheresis units (AU) from healthy volunteers were left untreated (non-IBS) or treated with the IBS following standard blood center procedure and storage at 22°C under agitation. Samples were analyzed for aggregation, P-selectin exposure, integrin activation, PS/PE exposure, platelet aggregation over collagen and adhesion to vWF at day (d) 1, 5, 7 and 10. Platelet survival was analyzed in NOD-SCID mice after 1 day storage by injection of fluorescently-labeled non-IBS or IBS platelets and analysis of blood at 0.5/2/5 hours post-injection. Bak expression was analyzed in platelet lysates by Western blotting.
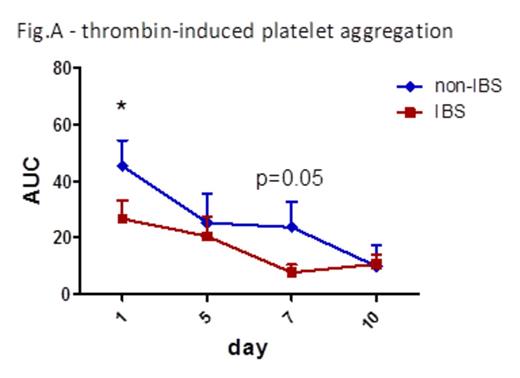
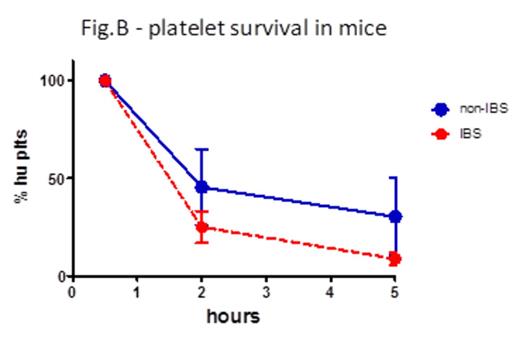
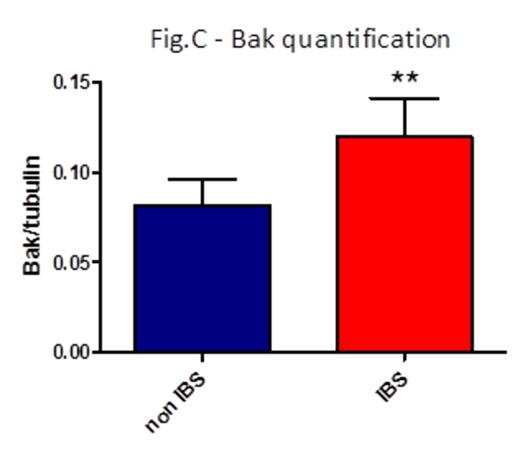
Results IBS treatment substantially reduced platelet aggregation already after 1 day storage (area under the curve -AUC- for thrombin 45.3 non-IBS vs 26.7 IBS, p=0.02, fig. A; AUC for collagen 19.1 non-IBS vs 4.9 IBS, p=0.07). P-selectin exposure and activation of a2bb3 were not different between non-IBS and IBS samples, as well as phosphatidylserine exposure as measured by Annexin V binding. Adhesion to vWF under high shear flow was reduced after 1 day storage, albeit not significantly due to some variability (platelet-covered area 4950 um2 non-IBS vs 1481 um2 IBS, p=0.2). Aggregation on collagen under flow was again markedly reduced upon IBS treatment at day 1 and 10 of storage (area d1: 67974 um2 non-IBS vs 35490 um2 IBS, p=0.15; d10: 37761 um2 non-IBS vs 14033 um2 IBS, p=0.13. AUC: 511426 non-IBS vs 247750 IBS, p=0.08). Platelet survival was reduced in NOD-SCID mice injected with IBS platelets compared to non-IBS platelets (% platelets in blood 2h post-injection: 45.5% non-IBS vs 25.1% IBS; 5h: 30.35% non-IBS vs 8.87% IBS; n=3, fig. B). Fluorescent platelets were quantified in spleen sections and found to be significantly higher in mice injected with IBS platelets (average platelet area: 19083 um2 non-IBS vs. 79395 um2 non-IBS, n=3, p=0.03). Analysis of Bak expression in platelet lysates revealed an increased amount of this pro-apoptotic mediator in IBS samples (Bak/tubulin integrated intensities: 0.08 non-IBS vs 0.12 IBS, p=0.007, fig. C), while there was no difference in the anti-apoptotic Bcl-XL.
Conclusions The IBS reduces platelet aggregation and responsiveness to physiological agonists in vitro, and platelet survival in the circulation of immunodeficient mice in vivo. This effect might be clinically relevant for patients receiving platelet transfusion and is possibly due to the induction of pro-apoptotic signaling in platelets by the method, which warrants further investigation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.