Abstract
Background: Rituximab (RXB) -containing chemotherapy forms the backbone of treatment for CD20 positive non-Hodgkin's lymphomas (NHL). RXB is combined with other agents as induction chemotherapy or as a single-agent maintenance therapy for indolent NHLs. RXB has been associated with reactivation of chronic hepatitis C virus (HCV) infection – a condition that leads to discontinuation of chemotherapy in 45% of patients who experience it. However, there is no data on the occurrence HCV reactivation according to the different types of RXB treatment strategies administered. We evaluated the effect of various RXB-containing regimens on HCV viremia.
Methods: Medical records of all patients with NHL seen at MD Anderson between 01/2008-05/2014 were screened. Records of those who were treated with RXB-containing regimens and had HCV RNA levels available before and after chemotherapy were analyzed. HCV reactivation was defined as an increase of HCV RNA of ≥ log10IU/mL over pre-chemotherapy levels. Acute exacerbation of HCV infection (AcEx) was defined as an increase of alanine aminotransferase (ALT) levels of ≥ 3 times upper normal limit in the absence of liver infiltration of cancer, hepatotoxic drugs, recent transfusion, and coinfections. The groups compared were those who received RXB + chemotherapy (induction), with those who received RXB + chemotherapy followed by RXB maintenance therapy. Categorical variables were compared using χ2 or Fisher's exact test and continuous variables were compared using Wilcoxon rank sum test. Within each group, continuous variables before and after chemotherapy were compared using Wilcoxon signed-rank test. All P values < 0.05 were considered statistically significant.
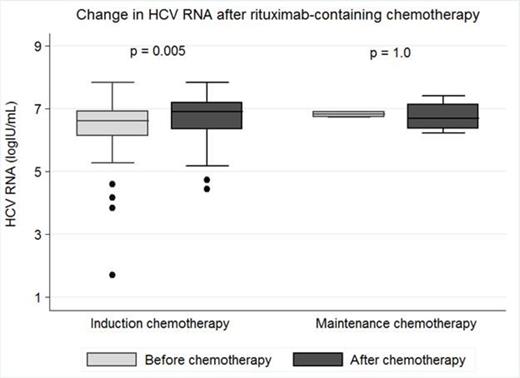
Results: We analyzed 28 HCV-infected patients (14 prospectively; 14 retrospectively) who received RXB-containing chemotherapy. Most patients were males (64%), whites (57%), and had a median age of 54.7 years (interquartile range, 47.8 – 61.4 years). Types of NHL included diffuse large B cell (54%), follicular (21%), marginal zone B cell (11%), mantle cell (4%), lymphoplasmacytic (4%), small lymphocytic (4%), and undetermined low grade B cell (4%) lymphomas; mostly Ann Arbor stage IV (82%). HCV genotype 1 was the most predominant strain (72%). Very few patients (12%) had cirrhosis when RXB chemotherapy was initiated. HCV treatment was administered before RXB chemotherapy in 9 (32%) patients but no one had resolved viremia (sustained virological response) at the time of cancer treatment. Overall, 43 courses of RXB-containing chemotherapy were administered, mostly as induction therapy (n = 39; 91%). Most common RXB-containing regimens administered were R-CHOP (n = 13; 30%), R-ICE (n = 6; 14%), and RXB with bendamustine (n = 5; 12%). In the entire cohort, when compared to pre-chemotherapy baseline levels, there were significant elevations in HCV RNA (median, log10IU/mL; 6.6 vs. 6.9; P = 0.005) and ALT (median, IU/L; 50 vs. 115; P < 0.0001) only after administration of RXB as induction chemotherapy, with no significant changes being found after RXB maintenance chemotherapy [HCV RNA (6.8 vs. 6.7; P = 1.0); ALT (37 vs. 59; P = 0.07)] (See Figure). When compared to maintenance therapy, those who received induction therapy attained peak HCV RNA (median, days; 590 vs. 140; P = 0.006) and peak ALT (median, days; 172 vs. 43; P = 0.02) earlier after RXB administration. HCV reactivation occurred only when induction chemotherapy was administered (n = 7). Doses of RXB-containing chemotherapeutic agents had to be modified (reduced or postponed) in 4 out of the 7 (57%) episodes of HCV reactivation. Median relapse-free survival for those who had dose modifications was lower than those without (14.6 months vs. 20.1 months; P = 0.13).
Conclusion: HCV reactivation occurs after administration of RXB as a part of induction, but not maintenance chemotherapy for NHL. This virologic condition is associated with dose modification of RXB-containing chemotherapeutic regimens in over one-half of cases.
Figure:
Torres:Gilead Sciences: Consultancy; Merck & Co., Inc. : Consultancy, Research Funding; Vertex Pharmaceuticals: Consultancy, Research Funding; Genentech,: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.