Abstract
Introduction: Ibrutinib (Imbruvica®), an oral, first-in-class covalent BTK inhibitor, is a new treatment option approved by FDA for chronic lymphocytic leukemia (CLL) patients (pts) with ≥1 prior therapy. We previously reported results from the phase III trial, in which ibrutinib (ibr) significantly improved progression-free and overall survival vs ofatumumab (ofa) in pts with relapsed/refractory CLL or small lymphocytic lymphoma (SLL) (Byrd et al. NEJM, 2014). Here, we report measures of patient well-being, including hematologic, immunologic, and quality of-life parameters, at interim analysis (IA).
Methods: Pts with CLL/SLL after ≥1 prior therapy were randomized 1:1 to ibr 420 mg/day until progression or unacceptable toxicity, or to ofa for up to 24 weeks. At IA, secondary efficacy endpoints of hematologic improvement (sustained improvement ≥56 days without transfusions or growth factors) and FACiT-Fatigue (FACiT-F) outcomes were analyzed, along with exploratory endpoints assessing disease-related symptoms (DRS), serum immunoglobulin, patient-reported outcomes (PROs) by EORTC QLQ-C30, and medical resource utilization (MRU).
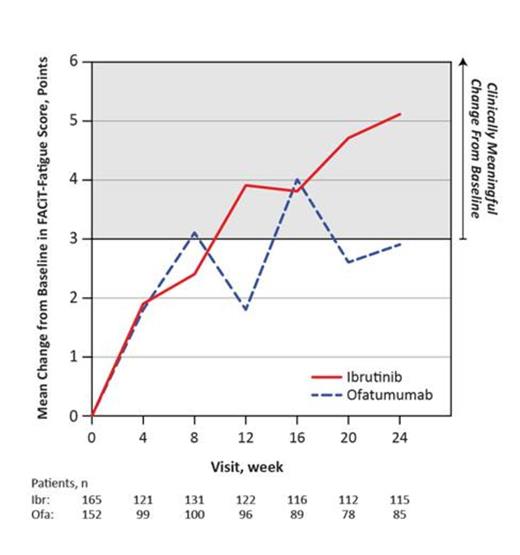
Results: Of 391 enrolled pts (ibr n=195; ofa n=196), 63% had cytopenia(s) at baseline: 45% had anemia (hemoglobin ≤11 g/dL), 35% thrombocytopenia (platelets ≤100×109/L), and 20% neutropenia (absolute neutrophil counts [ANC] ≤1.5×109/L). On the ibr arm, 69% of pts with baseline cytopenias experienced sustained improvement in blood counts compared to 43% on ofa (P<0.0001, table). There was no decrement in serum immunoglobulins (IgA, IgG, IgM) during follow-up. IgA levels increased on the ibrutinib arm only (mean change from baseline: 41% at week 24). Immune cells CD4+ and CD8+ T-cell and NK-cell levels remained relatively stable during treatment. Mean change in FACiT-F score from baseline are shown in the figure for ibr and ofa arms. At week 24, clinically meaningful (≥3 points) improvement in FACiT-F occurred in more pts on ibr than ofa (59% vs 46%, P=0.06); clinically meaningful deterioration was reported by 14% vs 24% (P=0.08), respectively. A larger proportion of ibr vs ofa pts showed clinically meaningful improvement on EORTC-QLQ-C30 global health scores (46% vs 40%). Clinically meaningful improvement (≥10 points) from baseline to week 24 in ibr vs ofa pts was observed for fatigue (median 11 vs 0). A 50% reduction in lymph node based on IRC-assessed CT was observed in 93% (177/190 evaluable) of ibr pts compared with 17% (29/174 evaluable) of ofa pts (P< 0.0001). Splenic response based on IRC-assessed CT was reported for 85% (138/163 evaluable) of ibr pts compared with 54% (82/151 evaluable) of ofa pts. Baseline DRS were comparable between groups; however, improvement by at least 1 grade (G) post-baseline was observed in ibr relative to ofa pts for weight loss (100% vs 93%), fatigue (88% vs 72%), night sweats (95% vs 86%), and anorexia (100% vs 64%), respectively. Cumulative MRU was comparable for growth factors and transfusions, with ibr pts having longer median treatment duration: 8.6 months for ibr vs 5.3 for ofa. Hospitalizations in the first 30 days occurred in 2% ibr vs 15% ofa pts. After adjusting for treatment exposure duration, diarrhea, grade 1-2 bleeding events (e.g petechiae), and atrial fibrillation were among those noted to be more frequent on the ibrutinib arm while events such as fatigue, infusion reaction, and peripheral sensory neuropathy were seen more commonly with ofa. Major hemorrhage events were uncommon, observed in 2 patients on the ibr arm vs 3 on ofa arm without adjusting for exposure time. Exposure-adjusted analysis showed no difference in any-grade infection and a 43% relative reduction for grade 3 or higher infections for ibr.
Conclusions: Ibr compared to ofa when administered to previously treated CLL/SLL pts led to improvements in hematologic function and disease burden. A survival benefit with ibr, together with sustained improvements in hematologic endpoints and PRO suggest that ibr enhances quality of life while prolonging survival.
Sustained hematologic improvement
| Parameter, n (%) . | ITT population . | Pts with cytopenias at baseline . | ||||
|---|---|---|---|---|---|---|
| Ibr n=195 | Ofa n=196 | P value | Ibr n=195 | Ofa n=196 | P value | |
| Platelets | 60 (31) | 19 (10) | <0.0001 | 53/74 (72) | 14/64 (22) | <0.0001 |
| ANC | 52 (27) | 19 (10) | <0.0001 | 26/41 (63) | 12/38 (32) | 0.0047 |
| Hemoglobin | 42 (22) | 32 (16) | 0.1883 | 42/89 (47) | 32/86 (37) | 0.1815 |
| Parameter, n (%) . | ITT population . | Pts with cytopenias at baseline . | ||||
|---|---|---|---|---|---|---|
| Ibr n=195 | Ofa n=196 | P value | Ibr n=195 | Ofa n=196 | P value | |
| Platelets | 60 (31) | 19 (10) | <0.0001 | 53/74 (72) | 14/64 (22) | <0.0001 |
| ANC | 52 (27) | 19 (10) | <0.0001 | 26/41 (63) | 12/38 (32) | 0.0047 |
| Hemoglobin | 42 (22) | 32 (16) | 0.1883 | 42/89 (47) | 32/86 (37) | 0.1815 |
Barrientos:Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Brown:Sanofi, Onyx, Vertex, Novartis, Boehringer, GSK, Roche/Genentech, Emergent, Morphosys, Celgene, Janssen, Pharmacyclics, Gilead: Consultancy. Kay:Genetech: Research Funding; Pharmacyclics: Research Funding; Hospira: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees. Coutre:Janssen, Pharmacyclics: Honoraria, Research Funding. Tam:Pharmacyclics and Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Mulligan:Roche, Abbvie: Consultancy, Honoraria. Jaeger:Janssen Cilag: Honoraria. Devereux:Pharmacyclics, Janssen: Consultancy, Honoraria. Robak:Janssen: Consultancy, Research Funding. Schuh:Roche, Gilead, GSK, NAPP, Celgene: Honoraria. Bloor:GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Dearden:Roche, GSK, Gilead, Janssen, Napp: Honoraria. Jones:Pharmacyclics: Consultancy, Research Funding. Kierschniak:Pharmacyclics, GmbH: Employment. Eckert:Pharmacyclics: Employment. Suzuki:Pharmacyclics: Employment. Hsu:Pharmacyclics: Employment. James:Pharmacyclics: Employment. Byrd:Pharmacyclics: Research Funding. Hillmen:Pharmacyclics, Janssen, Gilead, Roche: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.