Abstract
Introduction:
Thrombotic microangiopathy (TMA) is clinical syndrome characterized by microvascular thrombosis, consumptive thrombocytopenia and microangiopathic hemolytic anemia (MAHA). It is a feature of a number of clinical conditions, most prominently thrombotic thrombocytopenic purpura-hemolytic uremic syndrome (TTP-HUS) and rarely an initial manifestation of systemic malignancy.
Case:
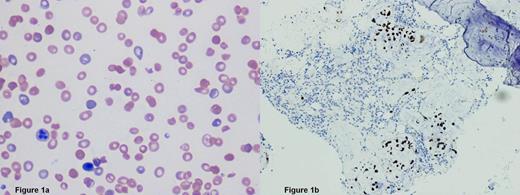
A previously healthy 31-year-old black woman presented to the emergency department with a 2 week history of lower back pain and hematuria and 1 week history of dyspnea on exertion and dizziness. She denied diarrhea, hematochezia, fever, or abdominal pain. Physical findings were unremarkable with normal blood pressure. Initial laboratory results revealed a white blood cell count of 4.4 x103/µL, hemoglobin of 4.4 g/dL, hematocrit 12.9%, platelets 43 x103/µL, absolute reticulocyte count 259.8 x103/µL, reticulocyte percentage 23.1%, haptoglobin <10 L mg/dL, LDH 1424 units/L, creatinine 0.7 mg/dL, total bilirubin 1.6 mg/dL, negative pregnancy test, and 10-15 white blood cells and >50 red blood cells per high powered field on microscopic urinalysis. Coombs test results were negative, the coagulation profile was normal, and peripheral blood smear revealed evidence of thrombocytopenia and MAHA (figure 1a). Diagnosis of TTP was made and treatment with daily therapeutic plasma exchange (TPE) was initiated. On hospital day 4, ADAMTS13 level drawn at diagnosis before initiation of plasma exchange returned at 94%, prompting further investigation as to the cause of her TMA. Serologic tests for hepatitis B & C virus, human immunodeficiency virus, antinuclear antibody (ANA), cardiolipin and beta-2 glycoprotein antibodies and computerized tomography (CT) scan of the abdomen and pelvis were all negative. Despite daily TPE for 6 days, she remained thrombocytopenic and continued to have ongoing hemolysis. Therefore, on hospital day 6, bone marrow aspiration and biopsy (figure 1b) was performed which showed a normocellular bone marrow for age (cellularity 70%) with full-spectrum trinlineage hematopoiesis, involved by metastatic signet-cell ring carcinoma. Immunostains were positive for CK7, CK20, CK 8/18, CDX2, pancytokeratin, and negative for PAX-8 and mammoglobin. TPE was discontinued and she was supported with packed red blood cell and platelet transfusions.
Positron emission tomography–computed tomography (PET-CT) revealed a heterogeneously enhancing mass in the midline superior anterior wall of the bladder measuring 2.7 x 5.1 cm, but without abnormal uptake on PET. She underwent cystourethroscopy and a transurethral resection of bladder tumor which revealed a 6 cm solid sessile mass. Final pathology confirmed the diagnosis of moderate to poorly differentiated invasive urachal adenocarcinoma with mucinous and signet-ring cell features.
Given the presence of metastatic disease, she was treated with a chemotherapy regimen consisting of cisplatin, fluorouracil, and leucovorin on days 1 through 5. Gemcitabine was held due to elevated liver transaminases. On day 1 of treatment, platelet count was 24 x103/µL and hemoglobin 8.1 g/dL. By treatment day 7, platelets had risen to 79 x103/µL. She last required a PRBC transfusion on treatment day 4.
Discussion:
TMA can occur in a variety of disorders, including TTP, HUS, malignant hypertension, scleroderma, antiphospholipid antibody syndrome, systemic lupus erythematosus, preeclampsia, HIV infection, and disseminated malignancy. Patients presenting with evidence of TMA and thrombocytopenia should elicit a high clinical suspicion for TTP and be urgently treated with plasma exchange. Deficiency of the von Willebrand factor-cleaving metalloproteinase ADAMTS13, or the presence of antibodies to ADAMTS13 plays a key role in the pathogenesis of primary TTP, although these findings are not necessary to make the diagnosis. However, patients with a high or normal ADAMTS13 level and without antibodies to ADAMTS13, especially those who do not improve with plasma exchange, should prompt an investigation for other causes of TMA. To our knowledge, we report the first case of metastatic urachal carcinoma presenting with features of MAHA and thrombocytopenia.
Conclusion:
TMA associated with malignancy is a rare, but potentially fatal condition. Recognition of this rare entity is critical to the initiation of appropriate treatment of underlying malignancy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.