Abstract
NF-kB plays important roles in immunity and oncogenesis, indicating that therapeutic targeting of this pathway could be beneficial in various clinical settings; however,an NF-kB-specific inhibitor does not exist in clinical practice to date. One approach toward development of such a compound is small-molecule-mediated direct inhibition of one or several members of the NF-kB family of transcription factors, a network that comprises five structurally related proteins including p50, p52, RelA, RelB and c-Rel. After screening of a library of 15,000 small molecules with a biochemical assay, we identified two scaffolds with inhibitory activity specific for the NF-kB subunit c-Rel. These scaffolds act as direct c-Rel inhibitors by modifying the conformation of the c-Rel protein, thus preventing DNA binding.
We previously reported that in vitro treatment of T cells with the thiohydantoin IT-603 induces c-Rel deficiency, resulting in suppression of T cell alloactivation without compromising T cell activation triggered by recognition of tumor-associated or viral antigens (Shono et al., Cancer Discovery, 2014). Here, we for the first time demonstrate in vivo efficacy of a c-Rel inhibitor treatment regimen in mouse models of graft-versus-host disease (GVHD) and graft-versus-lymphoma (GVL), as well as xenograft models of human B cell lymphomas, revealing that inhibition of c-Rel activity allows not only for suppression of GVHD while retaining GVL activity, but it also mediates promising anti-lymphoma effects.
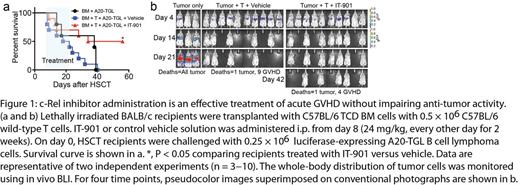
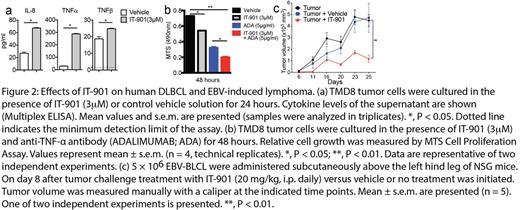
We first show that the novel small molecule IT-901 is a more potent c-Rel inhibitor than IT-603 and has a superior pharmacokinetic profile. IT-901 displayed significantly improved in vivo efficacy, ameliorating GVHD while preserving the anti-lymphoma activity of T cells (Figure 1a,b). Recent genetic evidence has established a pathogenetic role for NF-kB signaling in lymphoid malignancies. We therefore sought to explore the potential of IT-901 for targeted therapy of human lymphomas. We analyzed six representative diffuse large B cell lymphoma (DLBCL) cell lines including activated B-like (ABC; HBL1, TMD8, U2932) and germinal center B-like (GCB; Ly19, SU-DHL4, SU-DHL8) cell lines for nuclear translocation of c-Rel and found that c-Rel was constitutively active in all cell lines. To examine if c-Rel inhibition with IT-901 alters cytokine production by DLBCL cells, we analyzed cytokine levels in the supernatant after in vitro incubation with IT-901. IT-901 treatment resulted in decreased levels of a wide range of cytokines in TMD8 cells, with the notable exceptions of interleukin 8 (IL-8), tumor necrosis factor (TNF)-α, and TNF-β (P<0.05, Figure 2a). We next investigated if IT-901 treatment affected growth of DLBCL cells in vitro. We found that IT-901 dose-dependently inhibited cell growth of both ABC and GCB cell lines with IC50 values between 3µM to 4µM. Interestingly, IT-901 at a concentration of 3µM did not have an anti-proliferative effect on TMD8 cells, suggesting that cytokines such as IL-8 and TNF-α may be upregulated as a mechanism of resistance to c-Rel inhibition by activating alternative survival pathways. Indeed, in vitro treatment of TMD8 cells with a TNF-α neutralizing antibody inhibited cell growth, and this effect was enhanced when combining TNF-α blockade with c-Rel inhibition (P<0.01, Figure 2b). Furthermore, we detected high HMOX1 protein levels in DLBCL cells treated with IT-901, suggesting that HMOX1 expression was induced, which is a hallmark of oxidative stress. Indeed IT-901 induced production of high levels of reactive oxygen species in lymphoma cells. This suggests that induction of oxidative stress may be a second mechanism contributing to the anti-lymphoma activity of IT-901.
We next analyzed primary lymphoma cells and found that the c-Rel gene is widely expressed in human B cell malignancies and frequently amplified in DLBCL and EBV-transformed B cells. Importantly, intranuclear analysis of the c-Rel protein demonstrated that this transcription factor can be constitutively active in a wide range of human lymphomas. IT-901 efficiently inhibited growth of EBV-transformed B cells in vitro, and mediated significant anti-lymphoma activity in a xenograft model of EBV-induced lymphoma (P<0.01, Figure 2c).
In summary, our findings underscore multiple therapeutic benefits and great potential for clinical translation of a novel c-Rel inhibitor.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.