Abstract
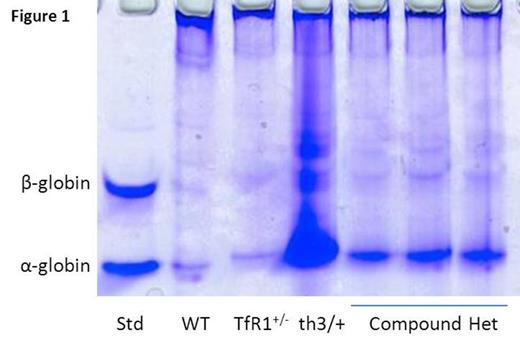
Transferrin receptor 1 (TfR1) is found in highest concentrations on erythroid precursors due to the disproportionately high iron requirement for hemoglobin synthesis, making transferrin-bound iron binding to TfR1 essential for erythropoiesis. Recent data reveals that TfR1 mRNA expression (6.48±2.23 vs. 1.0±0.25 relative to GAPDH, P=0.04 in sorted basophilic erythroblasts), whole cell protein concentration measured using ImageJ (11496±1783 vs. 1620±1448, P=0.0001 in reticulocytes), and cell surface concentration measured using flow cytometry (mean fluorescence index 17314±2370 vs. 11930±2530, P=0.002 in bone marrow basophilic erythroblasts) are increased in β-thalassemic (th1/th1) relative to wild type (WT) mice. We hypothesized that a relative decrease in TfR1 expression would improve the phenotype in β-thalassemic mice and crossed TfR1+/- (TfR1 heterozygote) mice [Levy JE Nat Gen 1999] with th3/+ mice, another commonly used mouse model of β-thalassemia. Of the 50 pups born, 13 had th3 genotype, 12 (92%) of which also contained the mutant TfR1, suggesting a strong survival advantage of TfR1 heterozygote th3/+ (compound heterozygotes) relative to th3/+ mice. Analysis of 3-4 month old compound heterozygotes revealed a significant decrease in splenomegaly (0.007±0.001 vs. 0.016±0.0041 g spleen/g body weight, P=0.0009), reticulocytosis (1019±186 vs. 1672±218 x 10^9 cells/uL, P=0.001), and α-globin precipitation on circulating RBCs (Figure 1 ) relative to th3/+ mice. Furthermore, compound heterozygotes exhibit improvement in circulating RBCs (12±0.1 vs. 9±0.6 x 10^6 cells/uL, P<0.0001) and hemoglobin (10±0.3 vs. 8.2±0.3 g/dL, P=0.0004) and decrease in MCH (8.9±0.2 vs. 10±0.2 pg, P=0.002) and non-heme liver iron (0.31±0.14 vs. 0.74±0.29 mg iron/g dry weight, P=0.02) relative to th3/+ mice. These findings suggest that decreased TfR1 expression results in more efficient erythropoiesis in β-thalassemia.
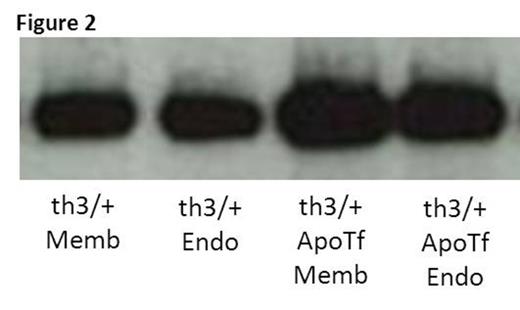
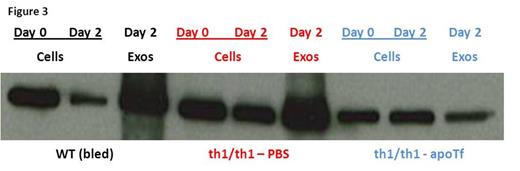
We previously demonstrate that exogenous apo-transferrin (apoTf) injections result in more circulating RBCs, increased hemoglobin, and reversal of splenomegaly in th1/th1 mice [Li H Nat Med 2010]. We hypothesize that ineffective erythropoiesis in th1/th1 mice is TfR1-mediated and involves excess iron delivery to erythroid precursors. To further explore the role of TfR1 in erythropoiesis, we evaluate apoTf-treated th1/th1 mice. TfR1 mRNA expression is unchanged in apoTf-treated relative to untreated th1/th1 mice despite more iron restricted erythropoiesis (MCH 24.56±0.72 vs. 33.98±1.67 pg, P<0.0001) and a significant decrease in serum soluble TfR1 [Liu J Blood 2013]. Western blots of reticulocytes from apoTf-treated th1/th1 mice reveal less TfR1 (4914±2561 vs. 11496±1783, P=0.006) and erythroid precursors from apoTf-treated th1/th1 mice analyzed by flow cytometry reveal more TfR1 (mean fluorescence index 24311±6025 vs. 11496±1783, P=0.02 in basophilic erythroblasts) relative to untreated th1/th1 mice. We hypothesized that TfR1 localization in sub-cellular compartments is altered in th1/th1 relative to WT mice and that increased apoTf enables normalization of TfR1 trafficking. Using differential centrifugation, we analyzed TfR1 in sub-cellular fractions in vivo and in vitro. Our results demonstrate a relative increase in membrane-associated and endosomal TfR1 in sorted bone marrow erythroid precursors from apoTf-treated relative to untreated th1/th1 mice. Furthermore, in vitro experiments also demonstrate increased membrane-associated and endosomal TfR1 in fetal liver cells from apoTf-treated relative to untreated th3/+ embryos (Figure 2 ). Lastly, we analyzed TfR1 exosomal release from reticulocytes after 2 days in culture, a commonly used method for exosome analysis, and demonstrate that exosomal release is decreased in reticulocytes from apoTf-treated relative to untreated th1/th1 mice (Figure 3 ).
Taken together, our data suggest that TfR1 plays a critical role in erythropoiesis, both in an iron-dependent and possibly independent capacity. We postulate that a defect in TfR1 trafficking, perhaps with a delayed or incomplete removal of TfR1 during erythroid differentiation, occurs in β-thalassemia, that reduction of TfR1 in β-thalassemic mice partially reverses ineffective erythropoiesis, and that exogenous apoTf decreases TfR1 expression and exosomal release while increasing membrane and endosomal cycling.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.