Abstract

Background
A pathogenic mechanism of Immune Thrombocytopenia (ITP) is insufficient platelet production by bone marrow megakaryocytes (MKs). MKs release platelets through a multistep process consisting of differentiation of MK progenitors, MK maturation, and formation of proplatelets, which in turn give rise to platelets. Many of these processes are primarily driven by thrombopoietin (TPO). However, the mechanism of proplatelet formation is not completely understood. It is controversial whether activation of apoptosis contributes to formation of proplatelets. Thrombopoiesis is generally increased in patients receiving TPO-receptor agonists (TPO-RA), therefore we hypothesized that MKs in these patients might show activation of apoptosis. This would support a role for apoptosis in thrombopoiesis in patients.
To test this hypothesis, we investigated activation of apoptosis in MKs isolated from ITP patients treated with TPO-RA, and in umbilical cord blood (UCB) derived MKs cultured in the presence of TPO-RA.
Materials and methods
Bone marrow aspirates of 16 chronic ITP patients (8 male and 8 female), median age 45.2 years old, were analyzed. Responders had at least 2 out of 3 recent platelet counts >503/µl. Samples were analyzed by flow cytometry (FACS) for Mitochondrial Outer Membrane Potential (MOMP) and PhosphatidylSerine (PS) in the CD41+ (MK) population. MKs positive for both MOMP and PS were considered apoptotic.
UCB CD34+ cells were cultured with Stemspan SFEM medium, 5 ng/ml Stem Cell Factor, and 50 ng/ml TPO (control). MKs were selected for assays on day 12 of culture by CD61 magnetic microbeads. Some cells were cultured with an additional 100 ng/ml of the TPO-RA Romiplostim (Romiplostim-treated) to mimic the presence of both endogenous TPO and TPO-RA in the human bone marrow. A third group of cells was cultured with 100 ng/ml TPO (2xTPO). Proplatelets were counted using inverted light microscopy on day 12 of culture in 10 random-field images. MOMP and PS externalization were measured by FACS. Caspase 3 and 7 activation, other markers of apoptosis, was measured by a Caspase 3/7 luminescence assay.
Results
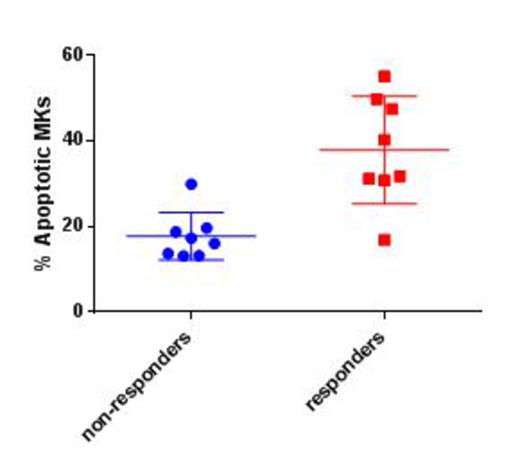
There was no significant difference in age, gender and splenectomy status between responders (N=8) and non-responders (N=8). The percentage of MKs was similar in all samples. FACS analysis revealed a significantly higher percentage of MOMP + PS positive apoptotic MKs in responders (mean 37.8±12.56) than in non-responders (17.7±5.53), p=0.02 (Figure 1). FACS analysis revealed a higher percentage of apoptotic cells in Romiplostim cultured UCB MKs than in control MKs (mean 57% versus 44%, p=0.03), and 2xTPO MKs (40%, p=0.02). No difference was found between 2xTPO and control cells (p=0.6)
Patient MKs did not grow in culture but MKs derived from UCB CD34+ cells cultured with Romiplostim were compared to control TPO MKs and 2xTPO MKs. While there was no difference in number of MKs per field between the 3 culture conditions; the number of proplatelets per MK was significantly higher in Romiplostim cultured vs. control (TPO) MKs (p<0.05). There was no significant difference between the number of proplatelets in 2xTPO MKs compared to Romiplostim cultured MKs. The caspase 3/7 assay showed higher activation of caspases in Romiplostim cultured MKs compared to both control (p=0.001) and 2xTPO MKs (p=0.03). There was no significant difference between 2xTPO and control MKs.
Conclusions
Our data show that in MKs from bone marrow aspirates obtained from ITP patients treated with TPO-RA, responders to treatment had a higher percentage of apoptotic MKs than non-responders. MKs derived from UCB cultured with the TPO-RA Romiplostim simultaneously exhibit increased proplatelet formation and increased markers of apoptosis activation compared to control or 2xTPO MKs. The association of apoptosis activation, response to therapy, and increased proplatelet production in culture suggest that apoptosis may play a role in proplatelet formation, at least in the presence of TPO-RA. These findings are contrary to certain studies in mice but in line with others that support the role of apoptosis in platelet production. Further studies in humans will be required to determine whether apoptosis plays a formative role in normal proplatelet formation.
Percentage of apoptotic MKs in non-responders and responders to TPO-RA.
Percentage of apoptotic MKs in non-responders and responders to TPO-RA.
Bussel:Sysmex: Research Funding; Shionogi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; Immunomedics: Research Funding; IgG of America: Research Funding; Genzyme: Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Research Funding; Cangene: Research Funding; Amgen Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Portola: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract