Abstract
Our previous findings have revealed the requirement of CCAAT Enhancer Binding Protein (C/EBPb), a leucine zipper transcription factor, in granulopoiesis (Hirai et al. Nat Immunol, 2006). During emergency situations such as infection, C/EBPb is involved in the sufficient supply of granulocytes through amplification of hematopoietic stem and progenitor cells (HSPCs) (Satake et al. J Immunol, 2012). In addition, we have shown that C/EBPb is upregulated by downstream signaling of BCR-ABL and promotes myeloid expansion and exhaustion of leukemic stem cells in chronic phase chronic myeloid leukemia (Hayashi et al. Leukemia, 2013). These observations suggested that C/EBPb plays important roles in regulation of normal and leukemic HSPCs. In this study, we focus on the functions of C/EBPb in normal HSPCs under stressed conditions.
At steady state, the frequencies of HSPCs in the bone marrow (BM) of C/EBPb knockout (KO) mice were identical to those in the BM of wild type (WT) mice. It suggests that C/EBPb has little impact on the emergence or maintenance of HSPCs during steady state.
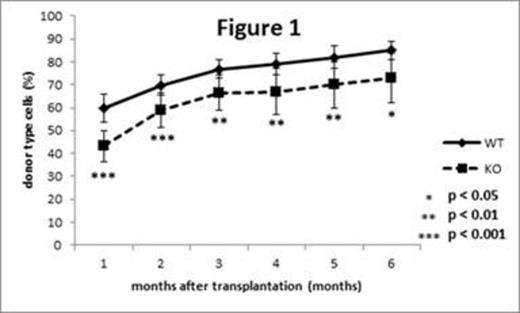
To investigate function of C/EBPb in HSPCs, competitive repopulation assay was performed. Total BM cells from either WT or KO mice (CD45.2+) and the equal number of competitor cells from the BM of CD45.1+ WT mice were transplanted into lethally irradiated recipient WT mice (CD45.1+), and the chimerism of CD45.2+ cells in the peripheral blood (PB) of the recipient mice was monitored once a month. Chimerism of KO cells in the recipient mice was significantly lower than that of WT cells at 1 month after transplantation (52.2 ± 10.3% vs 37.8 ± 8.8%, p < 0.0000001, n = 37 vs 36) and the differences were maintained thereafter (Figure 1), suggesting that C/EBPb is required at early time points after transplantation.
In order to elucidate the early events which make difference in the chimerism, homing ability was assessed first. Sixteen hours after transplantation of lineage depleted WT or KO BM cells (CD45.2+) together with lineage negative CD45.1+ WT BM cells, the frequencies of CD45.2+ WT and KO donor cells in the c-kit+ Sca1+ lineage- (KSL) fraction were identical. Then we compared the initial expansion of HSPCs. Purified 1000 KSL cells from either WT or KO mice (CD45.2+) were transplanted to lethally irradiated recipient WT mice (CD45.1+ / CD45.2+) together with the equal number of competitor KSL cells from WT mice (CD45.1+). The ratio of CD45.2+ KO cells to CD45.1+ competitors in the KSL fraction of the recipient mice was significantly lower than that of CD45.2+ WT cells at 4 weeks after transplantation (6.76 ± 2.35 vs 2.84 ± 1.16, p = 0.040, n = 4 vs 4). These results suggest that C/EBPb is required for initial expansion of HSPCs rather than for homing after transplantation.
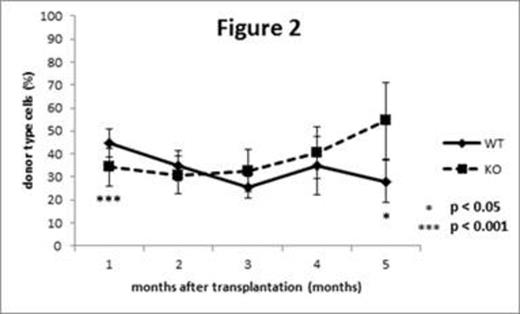
Next, we investigated the roles of C/EBPb in maintenance of HSPCs under stressed conditions. By staining of intracellular C/EBPb in combination with multi-color flow cytometric analysis, we found that C/EBPb is upregulated at protein level in KSL cells of WT mice 5 days after intraperitoneal injection of 5-fluorouracil (5-FU). Then the recipient mice were repetitively administered with 5-FU (150mg/kg i.p.) after BM transplantation in a competitive way. As mentioned above, the chimerism of KO cells in PB of recipient mice was significantly lower than those of WT mice at 1 month after transplantation. Interestingly, the chimerism of KO cells gradually increased by repetitive administration of 5-FU and even overtook those of WT cells 5 months after transplantation (Figure 2). In accordance with the changes observed in the PB, the chimerism of KO cells in the KSL fraction in the BM of recipient mice was significantly higher than those of WT cells (70.7 ± 25.3% vs 12.1 ± 9.78%, p = 0.016, n = 5 vs 4) 5 months after transplantation, suggesting that WT HSPCs exhausted earlier than KO HSPCs in response to hematopoietic stress.
From these findings, we conclude that C/EBPb is required for initial expansion and exhaustion of HSPCs after hematopoietic stresses. We are currently investigating the molecular targets of C/EBPb and its clinical significance in the pathogenesis of leukemia.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.