Abstract
Chronic lymphocytic leukemia (CLL) is a slowly progressing but incurable disease with high unmet medical needs.
As of 2014, there were few reliable publication sources of epidemiology data for CLL in France, particularly with regard to relapsed patient population. In addition, epidemiology registries provide useful data on disease incidence but no information can be directly obtained on the number of relapsed patients. At the same time, several new and effective drugs will be made available to the Hematologist/Oncologist community. As a consequence, there will be an increase in the complexity of therapeutic algorithms.
We undertook an innovative modeling approach to address the need for a greater understanding of the number of relapsed patients available to these new therapies. The objective of the model was to determine the yearly incidence of first line and later lines of therapy patients from 2014 to 2018 and in consequence, provide relevant data to help determine which population might be in need for the new compounds and bring accurate data that could be implemented into a prospective economic model.
First, we used the Registre Régional des Hémopathies Malignes de Basse-Normandie (RRHMBN) to collect real world patient characteristics, such as tumor staging (based on Binet Classification), fitness status, and deletion 17p. The RRHMBN is dedicated to Hematological malignancies and supported by official Health Authorities: Institut National du Cancer(INCA) and Institut National de Veille Sanitaire (INVS).
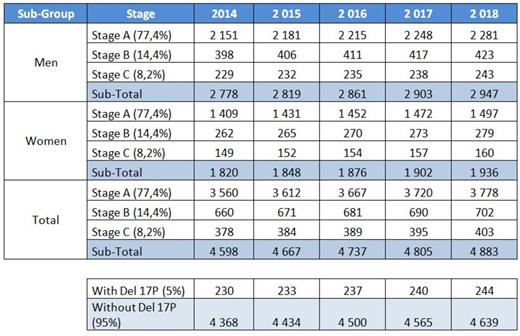
Following that, we applied these data to the French National Network of Registers FRANCIM incidence and survival data. 27.6% of patients were under 65 years. 50% of patients over 65 years had unfit status. 77% of patients had stage A, 14% stage B and 8% stage C. 2/3 of stage A were not treated and 1/3 evolved to stage B or C. 5% of patients had a 17p deletion at diagnosis (Table 1 below).
Table 1. Yearly number of patients newly diagnosed
Finally, we applied the results from medical literature to progression free survival for each standard of care treatment in our epidemiological model, Fludarabine (F) – Cyclophosphamide (C) – Rituximab (R) regimen with a PFS equal to 51.8 months for patients under 65 years. (R) – Bendamustine (B) regimen with a PFS equal to 33.9 months for patients fit and over 65 years. For unfit patients over 65 years, ofatumumab (O) – Chlorambucil (C) regimen with a PFS equal to 27 months.
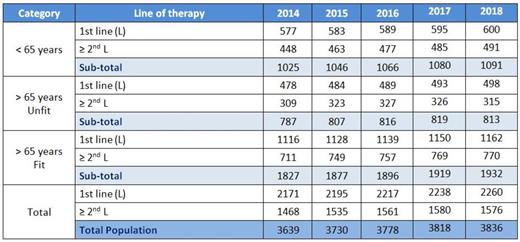
The table below (Table 2) shows the yearly number of patients eligible for treatment in first and subsequent lines of therapy as produced by our model.
Table 2. Yearly number of patients in need for treatment in first and subsequent lines
Our modeling approach compensates for a lack of real world information on relapsed CLL. We have shown that there is a significant number of relapsed patients who are in need of new treatments in France. The model is able to answer the question of how many patients are in need of novel therapies in later lines through 2018 and provide for CLL investigators efficient prospective data that can be integrated into medico-economic model development.
Furthermore, these data address a current medical need for the development of a new CLL algorithm for patient management, necessitated by the expected arrival of new treatment options for patients in the relapsed setting.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.