Abstract
Background: Single-agent carfilzomib (CFZ), a selective proteasome inhibitor, received accelerated approval in the US (July 2012) for the treatment of patients with relapsed and refractory multiple myeloma (MM). The approved dosing schedule is a 2–10 minute intravenous infusion on days 1, 2, 8, 9, 15, and 16 of a 28-day cycle. The starting dose is 20 mg/m2 during cycle 1; if well tolerated, it should be escalated to a target dose of 27 mg/m2 during cycles 2 and beyond, as CFZ dose-escalation may be associated with improved responses. To date, adherence to this schedule in a real-world setting has not been evaluated. We present results from a retrospective analysis that assessed the frequency of CFZ dose escalation in routine clinical practice and its association with patient demographics, clinical characteristics, concomitant medications, and duration of therapy (DOT).
Methods: This study was a retrospective analysis of clinical practice data with CFZ in the US. Data were obtained from the OSCER electronic medical record (EMR) database, which collates information on patient demographics, clinical characteristics, and treatment from multiple hematology/oncology practices. Patients were included if they were diagnosed after January 1, 2005 and received CFZ between July 20, 2012 and March 1, 2014 after previous exposure to bortezomib and an immunomodulatory agent. In the primary analysis, patients were classified as escalators if they received an increased dose of CFZ within 29 days (start of cycle 2) of initiation and non-escalators if they did not receive an increased dose of CFZ within 29 days. The Kaplan-Meier method with death as a competing risk and end of follow-up as right censored was used to compare DOT between escalators and non-escalators. To test the sensitivity of the analysis to the criteria for classifying patients by presence of dose escalation, calculations were repeated with patients classified as escalators if they received an increased dose of CFZ by the third cycle of treatment and non-escalators if they did not receive an increased dose of CFZ by the third cycle.
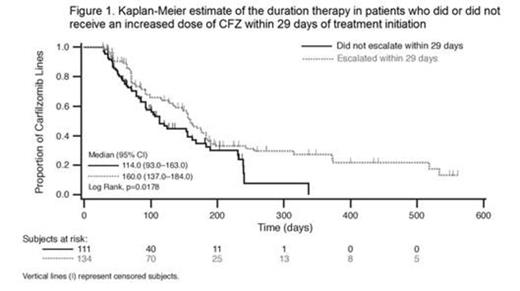
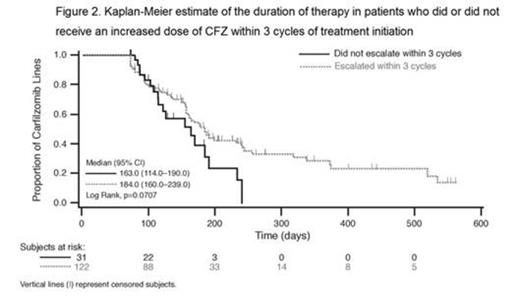
Results: A total of 281 patients in the OSCER EMR database met inclusion criteria. Data regarding dose escalation within 29 days or 3 cycles of starting treatment with CFZ was obtained for 244 and 153 patients, respectively; the majority (75%) were treated in practices in the southern region of the US. Fifty-five percent of patients received an increased dose of CFZ within 29 days of initiating treatment and 80% received an increased dose by the third cycle. Demographics (age and sex) and clinical characteristics (Eastern Cooperative Oncology Group performance status and presence of renal failure or chronic kidney disease) were similar between escalators and non-escalators (p>0.08). Although a greater proportion of non-escalators received granulocyte-colony stimulating factors (G-CSF) compared with escalators (<29 days: 16% vs 4%, respectively; <3 cycles: 19% vs 10%), a significant proportion of patients who did not receive G-CSF were also non-escalators (<29 days: 42%; <3 cycles: 18%). The median DOT was significantly higher in patients who were escalated within 29 days compared with patients who were not (160 vs 114 days, respectively; p=0.02; Fig. 1). Patients who were escalated within 3 cycles had a trend for increased median DOT compared to those who were not (184 vs 163 days, respectively; p=0.07; Fig. 2). Among patients who did not receive G-CSF, escalators had a longer DOT compared with non-escalators, consistent with results in the overall population.
Conclusion: A non-trivial proportion of patients (20%) who received CFZ were not dose-escalated by the third cycle of treatment. The demographics and clinical characteristics examined did not appear to distinguish escalators from non-escalators, and concomitant use of G-CSF did not fully explain why some patients were not escalated. As escalators had a longer DOT compared with non-escalators, there may be consequent differences in disease progression outcomes between escalators and non-escalators. Further investigation of demographics and disease characteristics (e.g. high-risk disease) in escalators and non-escalators is needed to understand the reasons behind the practice of continuing the starting dose, but not escalating, in those patients who do not receive target doses of CFZ, and what effect this practice has on disease progression outcomes.
Harvey:Onyx: Consultancy, Research Funding; Millennium: Consultancy, Research Funding; Novartis: Research Funding; Celgene: Research Funding; Acetylon: Research Funding; Amgen: Research Funding. Cong:Onyx Pharmaceuticals: Employment. Werther:Onyx Pharmaceuticals: Employment, Equity Ownership. Lethen:Amgen. Inc.: Employment. Lonial:Millennium: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Onyx: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.