Abstract
Background:
The aim of FVIII prophylaxis in Hemophilia A management is the reduction of risk for any bleed events (BE) in order to preserve long-term joint health and QOL. One proposed measure of the ideal prophylactic outcome is the virtual elimination of all BE not associated with trauma and/or of spontaneous etiology. Given the wide inter-patient variance in PK parameters, long-term prophylaxis outcomes and costs of therapy, PK-guided dosing has become an attractive approach to add more precision to dosing decisions and improve outcomes. Knowledge of the appropriate FVIII trough target for each individual patient to achieve a bleed-free outcome is needed however to design the ideal personalized PK-guided regimen.
Objective:
Model individual FVIII trough targets for achieving ideal outcomes in hemophilia A patients prescribed PK-guided prophylaxis every third day.
Methods:
FVIII infusion and BE records, and PK parameters from each subject participating in the 12-month PK-guided dosing arm of the randomized rAHF-PFM prophylaxis study (Valentino et al, 2012) were used to determine the predicted FVIII level at time of each BE. Median and maximum predicted FVIII values at time of spontaneous/non-trauma BE for all sites, and for joint and non-joint sites were estimated per subject. Number (%) of subjects with any spontaneous/non-trauma BE that occurred at predicted FVIII levels (maximum value and/or median value for those with more than one bleed) above and below specific theoretical FVIII trough values was modeled.
Results:
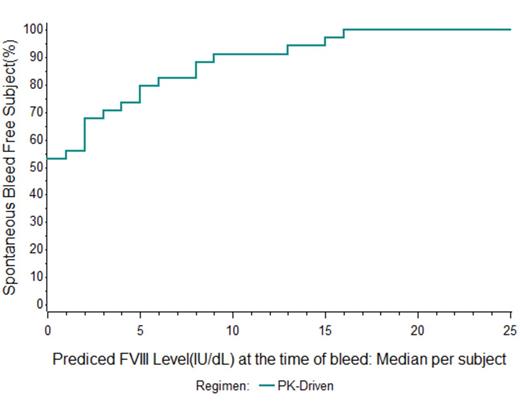
Of 34 subjects on PK-guided prophylaxis with rAHF-PFM targeting 1% trough every third day, 19 (56%) had no spontaneous BE during one year observation at FVIII levels (median) above the theoretical trough value of 1%. The number (%) of subjects with no spontaneous/non-trauma BE at FVIII levels (median) above the theoretical trough value of 3%, 5%, 10%, 15% and 20% levels increased to 71%, 79%, 31 91%, 97%, and 100%, respectively (Figure 1). All 57 spontaneous/non-trauma BE occurred at predicted FVIII levels ≤30%.
Conclusions: Evaluation of individual bleeding events and treatment records for this prophylaxis study population, most of which had pre-established hemophilic arthropathy, suggests that some patients may require higher trough targets to achieve an ideal outcome. Targeting a 1% trough FVIII level every 72 hrs was effective with 56% of subjects achieving a full year with no spontaneous BE. Modeling showed that targeting a 3 - 5% FVIII trough level would have led to more than 75% overall achieving zero spontaneous annual bleed rate (ABR), and that others may have benefitted from trough targets even as high as ≥15%. While this study could not necessarily account for or examine the role of differing levels of physical activity and/or arthropathy on levels of bleeding risk, modeling of FVIII levels post-infusion using individual PK parameters and infusion records along with the contemporaneous overlay of BE should provide valuable insight when designing the optimal, personalized prophylaxis regimen for patients.
Valentino LA, Mamonov V, Hellmann A, et al. J Thromb Haemost 2012 Mar; 10(3): 359-67.
Spotts:Baxter Healthcare Corporation: Employment, Equity Ownership. Pipe:Baxter: Consultancy, Honoraria. Berntorp:Baxter: Honoraria. Collins:Baxter: Consultancy, Honoraria. Oh:Baxter: Employment. Valentino:Baxter: Consultancy, Employment, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Biogen: Consultancy, Honoraria, Research Funding; CSL Behring: Consultancy, Honoraria, Research Funding; GTC Biotherapeutics: Consultancy, Honoraria, Research Funding; rEVO Biologics: Consultancy, Honoraria; Inspiration Bioscience: Consultancy, Honoraria, Research Funding; NovoNordisk: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract